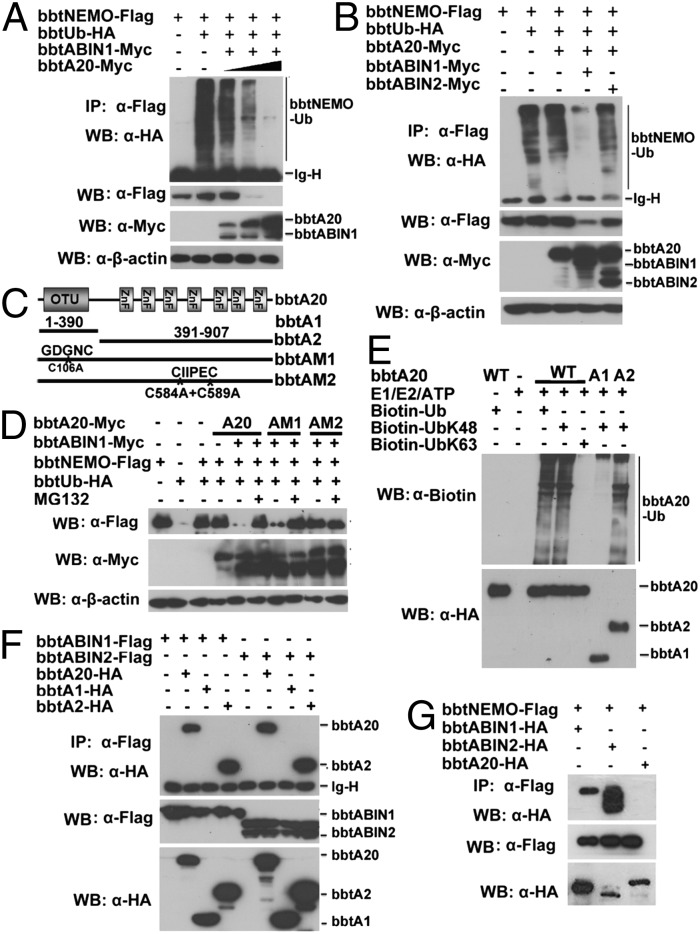
Fig. 4.
BbtA20 was physically linked to bbtNEMO to facilitate the K48-linked ubiquitination of bbtNEMO. (A) Cotransfection of bbtNEMO with bbtA20 and bbtABIN1 results in the degradation of bbtNEMO dependent on the dose of bbtA20. (B) Cotransfection of bbtNEMO with bbtA20 and bbtABIN1, but not with bbtA20, alone or together with bbtABIN2, results in the degradation of bbtNEMO. (C) Vectors of bbtA20 used in this study. (D) In vivo ubiquitination showed that point mutations within bbtA20 ZnF4, but not within bbtA20 OTU, attenuate the degradation of bbtNEMO. (E) In vitro analyses showed that bbtA20 can function as an E3 to catalyze the K48-linked ubiquitination, which is dependent on its ZnFs. (F) Transfected WT and the ZnFs only of bbtA20 bind bbtABIN1 and btABIN2. (G) Co-IP assays showed that both bbtABIN1 and bbtABIN2, but not bbtA20, can directly interact with bbtNEMO. All co-IP experiments were done at least twice.