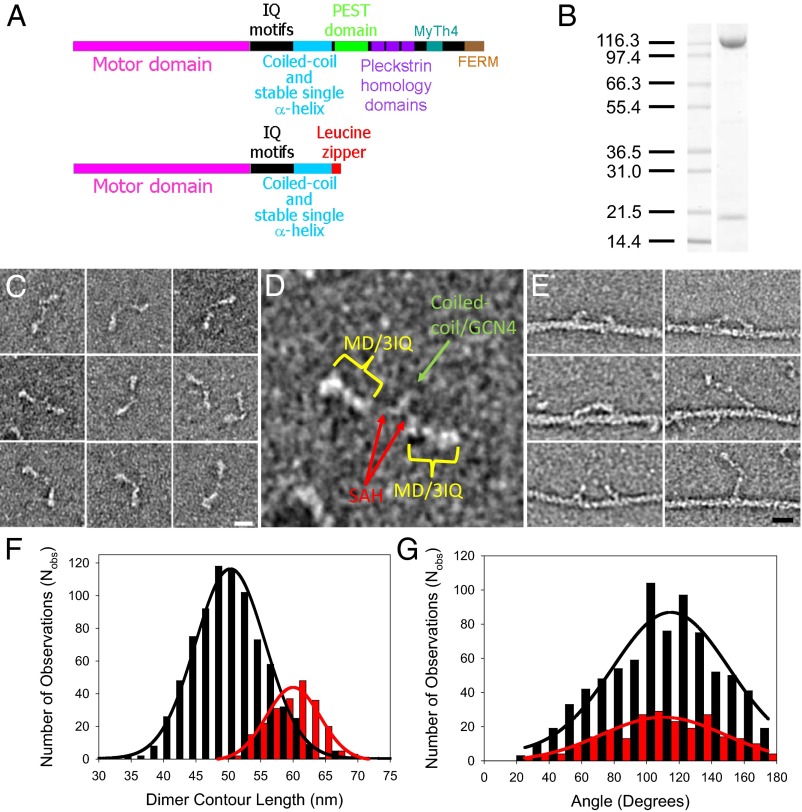
Fig. 1.
Myosin-10 heavy meromyosin-like (M10HMM) construct design. (A) Illustration of the full-length myosin-10 (Upper) and the M10HMM construct (Lower) structural organization. (B) Image of a typical SDS-polyacrylamide gel electrophoretogram (4–20%) of M10HMM after elution from an anti-FLAG resin. Approximately 0.4–0.8 mg of protein was purified per preparation. (C) Collage of M10HMM dimers negatively stained with 1% uranyl acetate (Each window = 92 × 92 nm). From electron micrographs it was determined that 87.2% of heavy chains were dimerized (Nobservations = 1,089). (Scale bar for all panels, 20 nm.) (D) An example M10HMM image from a panel from C with identification of regions, including motor domain (MD)/3 IQ motifs, the proximal coiled-coil region, and regions that most likely represents the stable SAH domains. Window size = 92 × 92 nm. (E) Images of M10HMM bound to actin in the presence of 1 μM ATP. Both single-headed binding and double-headed binding are seen. Examples can be seen in which the two heads of M10HMM span the actin pseudorepeat. (Scale bar for all panels, 20 nm.) (F) Histogram showing the contour length of M10HMM dimers (black bins), measured from the tip of one motor domain to the tip of the other [black line = Gaussian fit; peak = 51 ± 5.5 nm (SD); Nobs = 795; R2 = 0.99]. As a comparison, the contour length of myosin-5a-HMM (M5aHMM) (red bins) are shown [red line = Gaussian fit; peak = 60 ± 3.8 nm (SD); Nobs= 221; R2 = 0.96]. (G) Histogram showing the distribution of head-head angular variation of M10HMM (black bins; Nobs = 782) and M5aHMM (red bins; Nobs = 230) dimers. Gaussian fits showed peaks at 115 ± 2.7° (SEM) for M10HMM (black line; R2 = 0.89) and 110 ± 3.1° (SEM) (red line; R2 = 0.86) for M5aHMM.