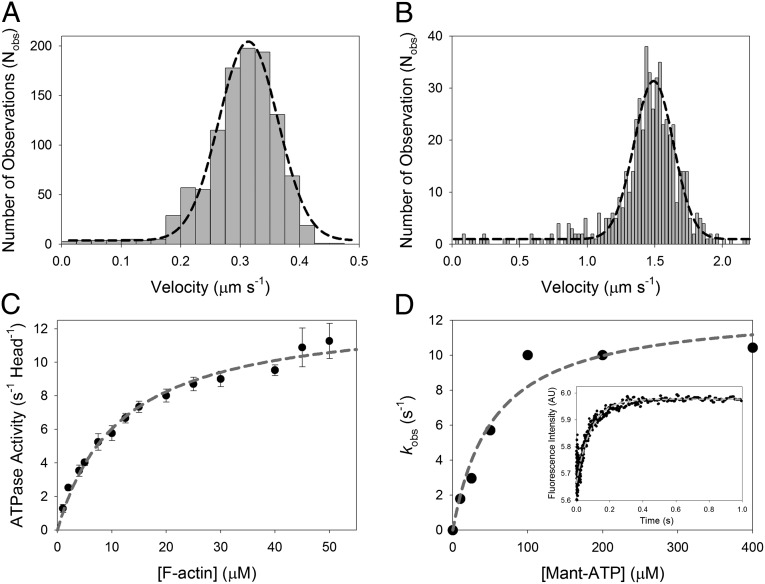
Fig. 2.
Ensemble mechanics and solution kinetics data of acto-M10HMM. (A) In vitro actin gliding assay using M10HMM construct at 23 °C. Average velocity = 310 ± 70 nm/s (SD) (Nfilaments tracked = 1,076). (B) In vitro actin gliding assay using M10HMM at 37 °C. Average velocity = 1,500 ± 120 nm/s (SD) (Nfilaments tracked = 541). (C) Plot of the actin-activated steady-state ATPase of M10HMM. In the presence of actin, the steady-state activity of M10HMM reached a maximal rate of 13.0 ± 0.47 s−1 (SD) (Vmax). Half-maximal activation was achieved at 12 ± 1.2 μM (SD) (KATPase) (Npreparations = 5). In the absence of actin, the basal steady-state activity of the construct was 0.04 ± 0.01 s−1 (SD). Data were collected at 25 °C. (D) To determine the ADP release rate from the acto-M10HMM complex, we used various mant-ATP concentrations to chase the dissociation of ADP from the M10HMM-ADP complex. A premixture of 1 μM M10HMM with 12 μM filamentous actin was incubated with 30 μM ADP. The acto-M10HMM-ADP complex was chased with different concentrations of mant-ATP. Fitting the kobs at different mant-ATP concentrations with a hyperbola was used to determine the maximal kobs, ∼12.7 s−1. (Inset) Transient record at 200 μM mant-ATP (postmixture concentration) with a kobs = 11.6 s−1. Data were collected at 25 °C.