Abstract
Here, the International Society for Stem Cell Research (ISSCR) Clinical Translation Committee introduces a series of articles outlining the current status, opportunities, and challenges surrounding the clinical translation of stem cell therapeutics for specific medical conditions.
Stem cells are valuable tools for the study of developmental biology, drug discovery, the development of diagnostics, and modeling and understanding disease, and they also show tremendous potential for the development of scientifically sound stem-cell-based therapies. The International Society for Stem Cell Research (ISSCR) is dedicated to the advancement of stem cell science and its applications, and the ISSCR Clinical Translation Committee, which consists of individuals who have a wide range of experience in areas where the clinical application of stem cells is either imminent or in place, has been given the twin mandate of (1) promoting clinical research and therapeutic application of stem cells, and (2) taking steps to prevent the exploitation of vulnerable patients receiving unproven stem cell treatments outside of well-regulated clinical trials. To help address the first of these mandates, we will publish, in the ISSCR section of Cell Stem Cell, a series of white papers that take a disease-specific approach to address particular concerns surrounding the study, feasibility, and efficacy of stem-cell-based treatments.
At present, physicians and scientists around the world are attempting to bring stem cells and stem-cell-derived products to clinical applications. The responsible development of such approaches requires the rigorous scientific investigation and testing of stem cells and their applications in both preclinical and clinical studies (see Figure 1). Moving “from bench to bedside” is complex and time-consuming, a reality often not evident to either the clinician or the patient given the rapid advancement of the stem cell field from a basic research perspective. Stem cells and their derivatives currently form the basis of therapies for treating a select number of conditions, including hematopoietic stem cell transplants for bone marrow diseases and the use of epithelial-stem-cell-based treatments for skin and corneal disorders and burns; and there are many clinical trials underway investigating the possible use of different cell types for a wide range of diseases. However, for most other conditions for which stem cells have the potential to be beneficial, they have not yet been shown to be so in appropriately conducted clinical trials.
Figure 1. Steps in the Translation of Stem Cell Research to Cell Therapeutics: From Bench to Bedside.
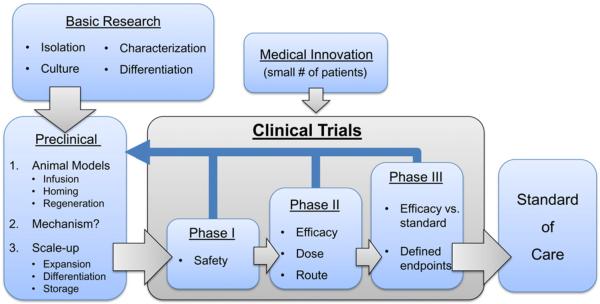
Translation begins with basic research to identify, isolate, culture, characterize, and differentiate the stem cell population of interest. Next, preclinical studies are needed to test strategies to reach appropriate therapies. This occurs in multiple steps: (1) the testing of function, using appropriate animal models if they exist, to evaluate the infusion, homing, and regenerative abilities of the cells of interest; (2) the determination (where possible) of the mechanism or mode of action underlying the treatment; and (3) the development of methods for the large-scale clinical-grade expansion and storage of these cells for clinical trials. Once rigorous preclinical testing has suggested an expected efficacy and often a mode of action or mechanism, the third step involves phased clinical trials that evaluate the safety, the route of administration in the appropriate patient population, and the efficacy of the treatment as per current Good Clinical Practice (GCP) guidelines. If the treatment is judged to be safe and effective and to offer advantages over existing treatments, it may be approved for and adopted as a standard of care. In this process, questions may arise that necessitate further preclinical studies. It must also be recognized that, in certain circumstances, very few patients (usually fewer than five) may be treated with innovative cell therapies outside of the classical clinical trial paradigm (“medical innovation”). If such treatments show encouraging results, they should be evaluated through a clinical trial process before becoming the standard of care. Innovation with advanced technologies can occur at all steps along the translation pathway.
To provide a framework for the responsible and timely development of clinically useful stem-cell-based treatments, the ISSCR Clinical Translation Task Force developed the “ISSCR Guidelines for the Clinical Translation of Stem Cells” (ISSCR, 2008). These guidelines provide a set of recommendations for researchers, clinicians, and oversight groups to aid their decision-making and include discussions on topics such as adoption of good manufacturing practices, importance and role of preclinical studies, and proper conduct and oversight of the clinical trial process. However, each disease or disorder that can potentially be treated by stem-cell-based treatments also presents a unique set of challenges and opportunities that cannot be reduced to a single set of guidelines. These disease-specific issues are the focus of this series of white papers. In this issue of Cell Stem Cell, we present the first article in the series describing the potential use of stem cells to treat neurodegenerative disorders.
For each area of application, we look at the approaches being used to translate stem cell science to the clinic, with a particular focus on the development of cell therapeutics. Each article will therefore aim to do the following:
provide a summary of our knowledge about the disease and the limitations of currently available therapies;
describe the preclinical models for their evaluation and limitations of the data available so far;
suggest what stem cells or derivatives could provide the most effective treatment and the need for their clear definition;
discuss the current state of clinical trials, including the requirements for a successful stem-cell-based therapy to be considered successful; and
explore potential directions for future investigation and suggest specific and prioritized steps that must be taken for the field to move forward.
By gathering a subset of leaders in areas where the clinical application of stem cells is envisioned, the goal of the ISSCR is to initiate action, foster debate, and provide a set of guideposts for moving the field forward. Our hope is that, by providing these starting points, we will be better positioned to shape, rework, and test stem cell applications to specific patient needs. It is through this process that we hope to foster the creation of effective stem cell treatments from the promise of stem cell science.
REFERENCE
- International Society for Stem Cell Research [Accessed January 13, 2012];Guidelines for the Clinical Translation of Stem Cells. 2008 http://www.isscr.org/clinical_trans/pdfs/ISSCRGLClinicalTrans.pdf.


