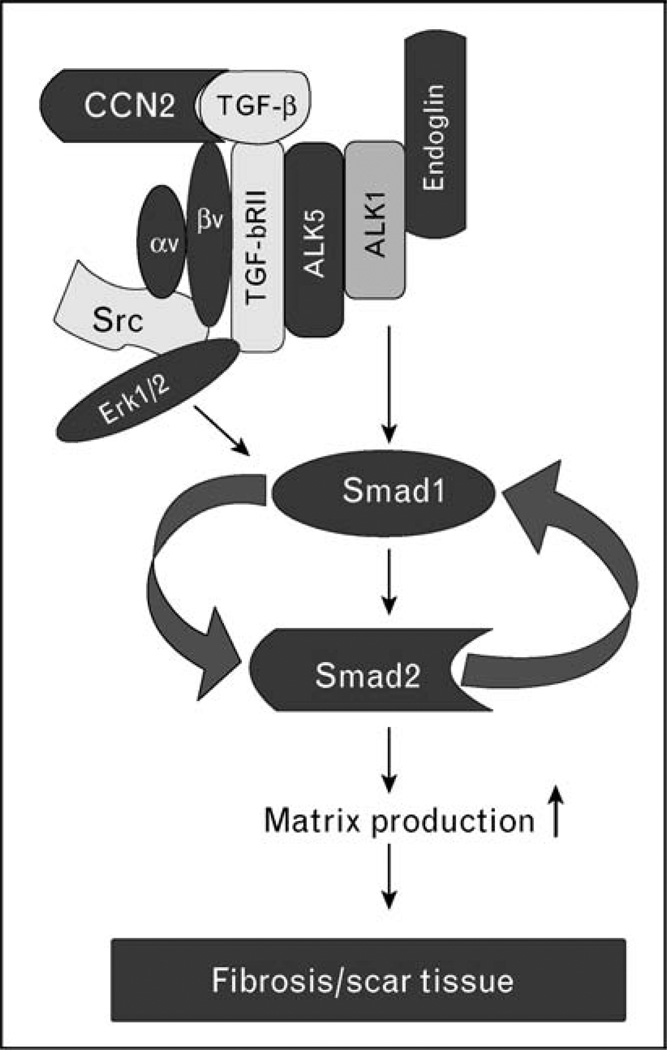
Figure 1. Activation of the autocrine CCN2–Smad1 signaling in scleroderma fibroblasts: a hypothetical model.
Activation of the Smad1 pathway requires cooperation between two ligands, transforming growth factor β (TGF-β) and CCN2, signaling through their cognate receptors. Elevated levels of endoglin [17] promote TGF-β signaling through a receptor complex also consisting of ALK5, ALK1, and TGF-β RII, which leads to induced phosphorylation of Smad1 [7,8]. Concurrent activation of ERK1/2 is required for Smad1 phosphorylation, although the specific role of ERK1/2 in this process has not yet been elucidated [8]. Binding of CCN2 to the αvβ3 integrin receptor activates ERK1/2 in a Src-dependent manner. As CCN2 has been shown to bind TGF-β and enhance TGF-β receptor binding and signaling [20], activation of Smad1/Erk1/2 signaling can occur in a spatially and temporally coordinated manner. Smad1 and Erk1/2 activate CCN2 gene expression leading to elevated CCN2 production, which then contributes to sustained activation of this pathway. Several components of this signaling pathway are expressed at elevated levels in scleroderma (SSc) fibroblasts, including ALK5, endoglin, αvβ3 integrin, Erk1/2, Smad1, and CCN2.