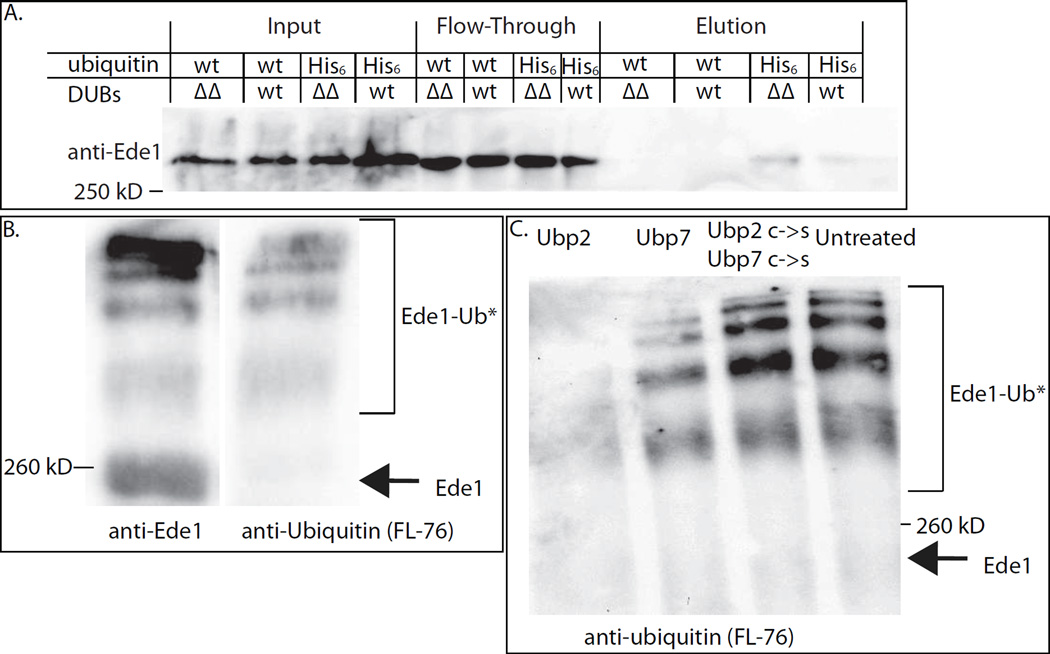
Figure 4. Evidence that Ede1 is de-ubiquitinated by Ubp2 and Ubp7.
(A) His6-ubiquitinated proteins were purified from UBP2 UBP7 (wt) and ubp2Δ ubp7Δ (ΔΔ) cells and the proteins eluted from nickel columns were immune-blotted to detect Ede1. More Ede1 was purified in ubp2Δ ubp7Δ cells, suggesting that Ede1 is more highly ubiquitinated in mutant cells. This experiment was repeated four times with similar results. (B) Equal volumes of immuno-isolated Ede1 were run on a 7% polyacrylamide gel and probed with either anti-Ede1 or an anti-ubiquitin (FL-76) antibody. Higher molecular weight bands correspond to ubiquitinated Ede1. (C) Immuno-isolated Ede1 was incubated at room temperature with GST-Ubp2, GST-Ubp7 or both GST-Ubp2 C745S and GST-Ubp7 C618S, and then separated on a 7% gel and probed with an anti-ubiquitin antibody (FL-76). The amount of ubiquitinated Ede1 was greatly reduced upon incubation with GST-Ubp2, somewhat reduced following incubation with GST-Ubp7 (which had less activity against Ub-AMC, Figure S3B) and was not deubiquitinated by the catalytically dead C->S mutants. See also Figure S3.