Abstract
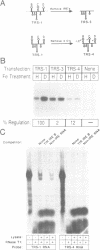
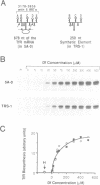
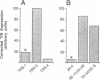
Post-transcriptional regulation of transferrin receptor mRNA levels by iron is mediated by a portion of the 3' untranslated region (UTR) of the mRNA. We have previously shown that a 678 nucleotide fragment of the 3'UTR contains the regulatory element(s). Within this region are five RNA structures which resemble the iron-responsive element (IRE) in the 5' untranslated region of the ferritin mRNA which is regulated translationally by iron. The IREs from the ferritin and transferrin receptor mRNAs compete in an in vitro assay for interaction with a cytoplasmic protein; the activity of this IRE-binding protein is dependent upon the iron status of the cells. Based on further deletion analysis reported here, the sequence required for iron regulation of the transferrin receptor have been limited to 250 nucleotides which we have produced synthetically and cloned. This sequence, which contains three IREs, is capable of producing iron-dependent regulation of transferrin receptor levels. Removal of the three IREs from the synthetic element results in loss of iron regulation. Moreover, deletion of a single cytosine residue from each of the three IREs in the synthetic regulatory element eliminates high-affinity binding to the IRE-binding protein in vitro and results in low levels of iron-independent transferrin receptor expression, consistent with production of a constitutively unstable mRNA. These data indicate that the ability of the mRNA to interact with the IRE-binding protein is required for regulation of transferrin receptor mRNA levels by iron.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


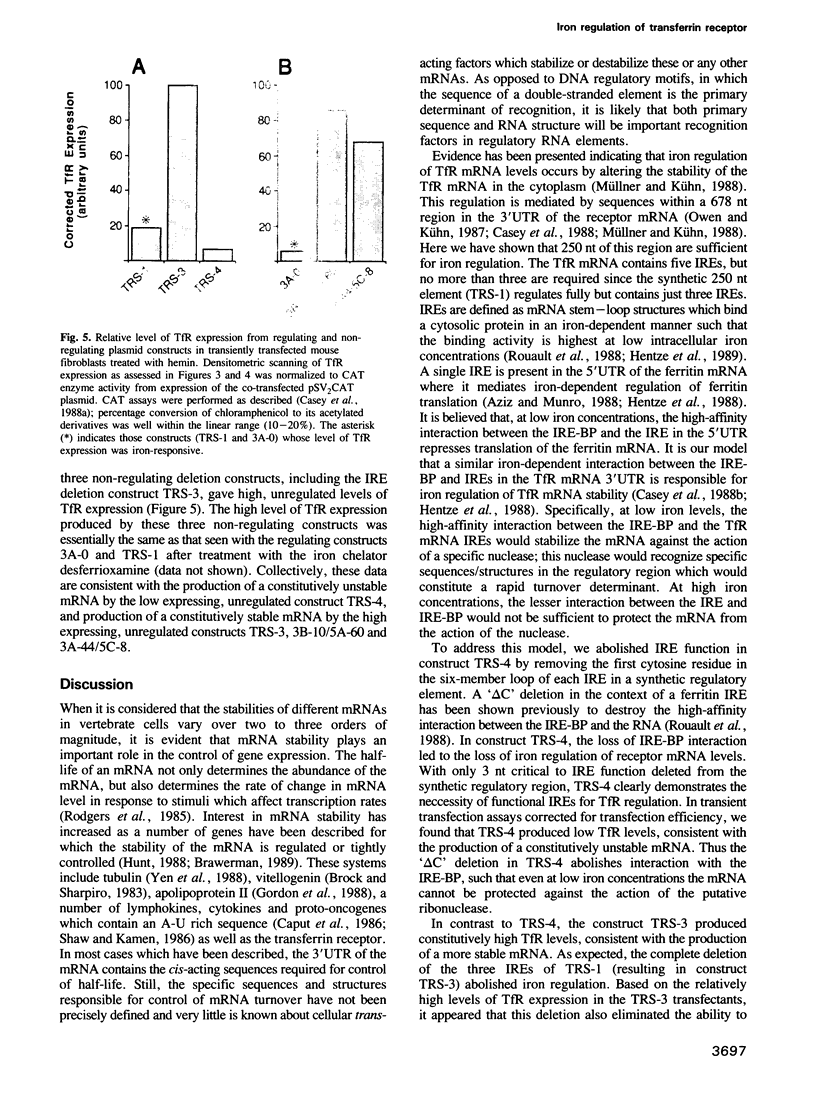


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brawerman G. mRNA decay: finding the right targets. Cell. 1989 Apr 7;57(1):9–10. doi: 10.1016/0092-8674(89)90166-9. [DOI] [PubMed] [Google Scholar]
- Brock M. L., Shapiro D. J. Estrogen stabilizes vitellogenin mRNA against cytoplasmic degradation. Cell. 1983 Aug;34(1):207–214. doi: 10.1016/0092-8674(83)90151-4. [DOI] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J. L., Di Jeso B., Rao K., Klausner R. D., Harford J. B. Two genetic loci participate in the regulation by iron of the gene for the human transferrin receptor. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1787–1791. doi: 10.1073/pnas.85.6.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J. L., Hentze M. W., Koeller D. M., Caughman S. W., Rouault T. A., Klausner R. D., Harford J. B. Iron-responsive elements: regulatory RNA sequences that control mRNA levels and translation. Science. 1988 May 13;240(4854):924–928. doi: 10.1126/science.2452485. [DOI] [PubMed] [Google Scholar]
- Caughman S. W., Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. The iron-responsive element is the single element responsible for iron-dependent translational regulation of ferritin biosynthesis. Evidence for function as the binding site for a translational repressor. J Biol Chem. 1988 Dec 15;263(35):19048–19052. [PubMed] [Google Scholar]
- Chan L. N., Grammatikakis N., Banks J. M., Gerhardt E. M. Chicken transferrin receptor gene: conservation 3' noncoding sequences and expression in erythroid cells. Nucleic Acids Res. 1989 May 25;17(10):3763–3771. doi: 10.1093/nar/17.10.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. A., Shelness G. S., Nicosia M., Williams D. L. Estrogen-induced destabilization of yolk precursor protein mRNAs in avian liver. J Biol Chem. 1988 Feb 25;263(6):2625–2631. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Casey J. L., Koeller D. M., Rouault T. A., Harford J. B., Klausner R. D. A model for the structure and functions of iron-responsive elements. Gene. 1988 Dec 10;72(1-2):201–208. doi: 10.1016/0378-1119(88)90145-x. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Rouault T. A., Barriocanal J. G., Dancis A., Harford J. B., Klausner R. D. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science. 1987 Dec 11;238(4833):1570–1573. doi: 10.1126/science.3685996. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Oxidation-reduction and the molecular mechanism of a regulatory RNA-protein interaction. Science. 1989 Apr 21;244(4902):357–359. doi: 10.1126/science.2711187. [DOI] [PubMed] [Google Scholar]
- Hunt T. Controlling mRNA lifespan. Nature. 1988 Aug 18;334(6183):567–568. doi: 10.1038/334567a0. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Ashwell G., van Renswoude J., Harford J. B., Bridges K. R. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeller D. M., Casey J. L., Hentze M. W., Gerhardt E. M., Chan L. N., Klausner R. D., Harford J. B. A cytosolic protein binds to structural elements within the iron regulatory region of the transferrin receptor mRNA. Proc Natl Acad Sci U S A. 1989 May;86(10):3574–3578. doi: 10.1073/pnas.86.10.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattia E., Rao K., Shapiro D. S., Sussman H. H., Klausner R. D. Biosynthetic regulation of the human transferrin receptor by desferrioxamine in K562 cells. J Biol Chem. 1984 Mar 10;259(5):2689–2692. [PubMed] [Google Scholar]
- Müllner E. W., Kühn L. C. A stem-loop in the 3' untranslated region mediates iron-dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell. 1988 Jun 3;53(5):815–825. doi: 10.1016/0092-8674(88)90098-0. [DOI] [PubMed] [Google Scholar]
- Owen D., Kühn L. C. Noncoding 3' sequences of the transferrin receptor gene are required for mRNA regulation by iron. EMBO J. 1987 May;6(5):1287–1293. doi: 10.1002/j.1460-2075.1987.tb02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicci P. G., Tabilio A., Thomopoulos P., Titeux M., Vainchenker W., Rochant H., Testa U. Hemin regulates the expression of transferrin receptors in human hematopoietic cell lines. FEBS Lett. 1982 Aug 23;145(2):350–354. doi: 10.1016/0014-5793(82)80198-1. [DOI] [PubMed] [Google Scholar]
- Rao K., Harford J. B., Rouault T., McClelland A., Ruddle F. H., Klausner R. D. Transcriptional regulation by iron of the gene for the transferrin receptor. Mol Cell Biol. 1986 Jan;6(1):236–240. doi: 10.1128/mcb.6.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers J. R., Johnson M. L., Rosen J. M. Measurement of mRNA concentration and mRNA half-life as a function of hormonal treatment. Methods Enzymol. 1985;109:572–592. doi: 10.1016/0076-6879(85)09116-9. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Caughman S. W., Harford J. B., Klausner R. D. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988 Sep 2;241(4870):1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Ward J. H., Kushner J. P., Kaplan J. Regulation of HeLa cell transferrin receptors. J Biol Chem. 1982 Sep 10;257(17):10317–10323. [PubMed] [Google Scholar]
- Yen T. J., Machlin P. S., Cleveland D. W. Autoregulated instability of beta-tubulin mRNAs by recognition of the nascent amino terminus of beta-tubulin. Nature. 1988 Aug 18;334(6183):580–585. doi: 10.1038/334580a0. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]