Supplemental Digital Content is available in the text.
Key Words: acute lymphoblastic leukemia, acute myeloid leukemia, bendamustine, dose-ranging study, overall response rate
Abstract
This open-label, single-arm, phase I/II, dose-escalation study was designed to determine the recommended phase II dose (RP2D), pharmacokinetics, tolerability, and efficacy of bendamustine in pediatric patients (age ranging from 1 to 20 y) with histologically proven relapsed/refractory acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML). Patients (27 with ALL, 16 with AML) received intravenous bendamustine on days 1 and 2 of each treatment cycle. Phase I involved planned dose escalation of bendamustine to establish the RP2D for phase II. Objectives included overall response rate, duration of response, and tolerability. Eleven patients were treated in phase I, and the RP2D was 120 mg/m2. In phase II, 32 patients received bendamustine 120 mg/m2. Two patients with ALL (bendamustine 90 mg/m2) experienced complete response (CR). Among patients who received bendamustine 120 mg/m2, 2 experienced partial response (PR); 7 had stable disease. The overall response rate (CR+CR without platelet recovery [CRp]) was 4.7% and biological activity rate (CR+CRp+PR) was 9.3%. No AML patients responded. The most common adverse events were anemia, neutropenia, thrombocytopenia, pyrexia, nausea, vomiting, and diarrhea. Bendamustine monotherapy has acceptable tolerability in heavily pretreated children with relapsed/refractory ALL or AML and appears to have some activity in ALL, warranting further studies in combination trials.
Despite ongoing improvements in outcomes for children with acute leukemias, relapses continue to occur and the prognosis for these patients remains poor. Thus, new treatments are needed for patients whose leukemia progresses or recurs following established therapies. Bendamustine is a bifunctional mechlorethamine derivative containing a benzimidazole heterocyclic ring. Mechlorethamine and its derivatives are alkylating agents that induce DNA damage, resulting in apoptosis.1 Although the exact mechanism of action of bendamustine and the role of the benzimidazole ring have not been fully defined, bendamustine is active against both quiescent and dividing cells, and treatment of tumor cells with bendamustine results in a large number of DNA double-strand breaks, consistent with its classification as an alkylating agent.2 However, bendamustine is distinct from other alkylating agents in that it possesses additional antitumor activity because of its unique composition.3 Bendamustine has demonstrated activity in adults with chronic lymphocytic leukemia and rituximab-refractory non-Hodgkin lymphoma (NHL).4,5 However, no data with regard to the use of bendamustine in children or in childhood acute leukemia have been reported.
In vitro, bendamustine has been shown to have activity against 2 pediatric acute lymphoblastic leukemia (ALL) T-cell (CCRF-CEM) and B-cell (CCRF-SB) lines at concentrations of 18 and 20 µM, respectively (data on file, Teva Branded Pharmaceutical Products R&D Inc.). Bendamustine has also demonstrated cytotoxic activity in the acute myeloid leukemia (AML) cell line HL-60, albeit to a lesser extent than cell lines of lymphoid origin.6 These results suggest that bendamustine may provide a benefit in acute childhood leukemia that warrants studying in the pediatric population.
The present study is an open-label, single-arm, phase I/II, dose-escalation study to determine the recommended phase II dose (RP2D), pharmacokinetics, and tolerability profile, as well as efficacy of bendamustine as a single agent in heavily pretreated pediatric patients with relapsed or refractory acute leukemia.
MATERIALS AND METHODS
Study Design
This international study was conducted in accordance with the Good Clinical Practice: Consolidated Guidance approved by the International Conference on Harmonisation and all applicable national and local laws and regulations. Patients received bendamustine infused intravenously over 60 minutes on days 1 and 2 of each 21-day cycle. Delays of up to 2 weeks were allowed for neutrophil and platelet recovery, for a maximum cycle duration of 35 days. Clinical response and hematologic recovery were assessed using International Working Group 2003 criteria (morphologic leukemia-free state) with bone marrow evaluation (Fig. 1).7
FIGURE 1.
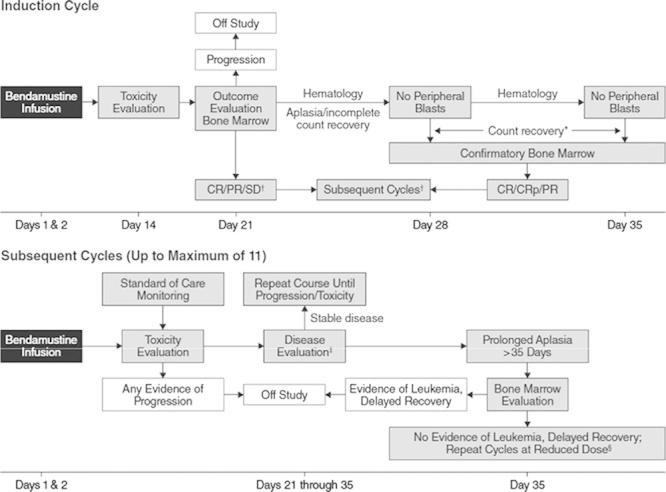
Treatment and evaluation plan. *All subsequent cycles require clinical determination of patient benefit. †Count recovery for induction defined as neutrophil count ≥1.0×109/L and platelet count ≥100×109/L. ‡Disease evaluation may include peripheral laboratory testing and/or bone marrow evaluation at the discretion of the treating physician. Count recovery for subsequent cycles defined as platelet count not requiring transfusion and neutrophil count ≥500. §Clinical discretion for additional cycles of therapy is required based on patient’s clinical need; maximum of 35 days for recovery, minimum neutrophil count is 500, and platelet count may be supported with transfusion. CR indicates complete response; CRp, complete response without platelet recovery; PR, partial response; SD, stable disease.
The study consisted of 2 phases. In phase I (dose-escalating), a starting dose of bendamustine 90 mg/m2 was used in the first cohort of 3 patients, with planned escalation to 120 mg/m2 and potentially to 150 mg/m2 in subsequent cohorts of patients. Clearance in adults aged 31 to 84 years does not vary by patient’s age,8 and it was expected that the clearance will be similar among children. Therefore, the initial dose was selected on the basis of the metabolic profile of bendamustine in adults.
In this 3+3 study design, each cohort could be expanded to up to 6 patients based on National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 toxicity.9 Dose-limiting toxicity (DLT) was defined as any nonhematologic NCI CTCAE grade 4 toxicity or any grade 3 skin rash or allergic reaction (hematologic events were not considered DLTs because of the nature of patients’ cancers). Bendamustine 150 mg/m2 was to be implemented only if the 120 mg/m2 dose was acceptably tolerated but resulted in subtherapeutic plasma levels when compared with data obtained from adults. In the event that ≥2 patients in the 90 mg/m2 cohort experienced a DLT, the bendamustine dosage was to be reduced to 60 mg/m2. The RP2D was defined as the dose one step below the dose at which ≥2 patients experienced a DLT. In phase II, additional patients were enrolled at the RP2D determined in phase I. Patients were followed until disease progression, withdrawal due to intolerability or other reasons, loss to follow-up, or completion of maximum of 12 cycles of therapy. After the end of treatment, patients were evaluated every 3 months for 12 months after the last dose, or until progression, death, or start of new cancer treatment.
Patients
Children aged 1 to 20 years with histologically proven (>5% blasts on morphologic assessment) ALL or AML with ≥1 relapse or refractory to the prior treatment and without the opportunity for potentially curative therapy were eligible to participate in this study. Nonhematologic toxic effects of prior therapy (ended ≥2 wk before first dose of study drug) were required to have resolved to grade 0 to 2 according to the NCI CTCAE version 4.0. Inclusion criteria included adequate liver function (bilirubin ≤1.5 times the upper limit of normal [ULN] and aspartate aminotransferase [AST] and alanine aminotransferase [ALT] ≤5 times the age-appropriate ULN) and renal function (serum creatinine <2 times the ULN) and Karnofsky or Lansky performance status ≥60. Previous hematopoietic stem cell transplant (HSCT) was allowed, but a patient’s last myelosuppressive therapy had to have ended at least 2 weeks before the first dose of the study drug. Patients with child-bearing potential were required to agree to practice birth control during the duration of the study and for 30 days after the end of treatment. Written and dated informed consent was obtained from patients or the parent(s) or guardian(s) of minor patients, or as required by local regulations. Patients were excluded if they were currently receiving any other systemic cancer treatment; had any active, uncontrolled systemic infection; severe concurrent disease; symptomatic untreated central nervous system involvement (concurrent systemic treatments were not allowed during the study); active graft-versus-host disease; known human immunodeficiency virus or active hepatitis B or C infection; pregnancy or lactation; or any serious uncontrolled medical or psychologic disorder that would impair the ability of the patient to receive the study drug. Patients treated at any dose of bendamustine were included in the evaluation for safety and efficacy, and pharmacokinetic analysis included all patients eligible for safety analysis who had valid pharmacokinetic data.
Assessments
The primary objective of phase I was to establish the RP2D; efficacy was a secondary assessment. Efficacy was also measured for patients who participated in phase II. The primary efficacy measure was overall response rate (ORR), defined as a composite of complete response (CR) and CR without platelet recovery (CRp) among patients enrolled at the RP2D in both phases at any cycle. The best response was assessed through the study (cycle 1: days 21 [% blasts on complete blood count] and 35 [bone marrow morphologic assessment]; subsequent cycles: complete blood count and differential unless prolonged aplasia required repeat bone marrow examination). CR required no evidence of circulating blasts or extramedullary disease, an M1 marrow (≤5% bone marrow blasts), absolute neutrophil count of 1.0×109/L or greater, and platelet count of 100×109/L or greater.7 Additional efficacy outcomes included ORR for phase II only, duration of response (DOR) in patients who achieved CR or CRp, and biologic activity (at least a partial response [PR], defined as complete disappearance of circulating blasts, an M2 marrow [5% to 25% bone marrow blasts], or appearance of normal progenitor cells or an M1 marrow that did not qualify for CR or CRp) in patients enrolled in either phase. Tolerability assessments included adverse events, clinical laboratory values, concomitant medication throughout treatment, and vital signs.
Pharmacokinetics
Pharmacokinetic samples to determine the plasma concentrations of bendamustine were obtained from all patients in phase I or II during cycle 1 only. Samples were obtained before bendamustine infusion and at preselected time points at 3, 6, 10 (±2), and 24 hours. The 24-hour postinfusion sample was obtained before the start of the infusion on day 2. Further pharmacokinetic sampling was not done after 24 hours because, with the short half-life and low plasma concentrations observed by 12 hours after bendamustine infusion in adults, plasma concentrations were expected to be nonquantifiable or negligible beyond 24 hours in this pediatric population.8
After collection, blood samples were inverted 5 to 10 times to mix the contents, and the tubes were immediately placed into an ice bath. Plasma was harvested by low-speed centrifugation (2000 rpm, ∼10 min, 4°C) within approximately 30 minutes of sampling. Samples were then shipped on dry ice to BASi, West Lafayette, IN, for analysis. Concentrations of bendamustine were determined by BASi using a validated high-performance liquid chromatography method with tandem mass spectrometric detection. The quantifiable range of the assay was from 0.10 to 100.00 ng/mL for bendamustine.
Pharmacokinetic parameters calculated for bendamustine and its metabolites included maximum observed plasma drug concentration (Cmax), time to maximum drug concentration (tmax), area under the plasma drug concentration by time curve from time 0 until the last measurable plasma concentration (AUC0-t), and area under the plasma drug concentration by time curve from time 0 until 24 hours after study drug administration (AUC0-24). Pharmacokinetic data from adults were used for comparison.
Statistics
The primary analysis group for efficacy included all patients who received the RP2D in phases I and II; efficacy was also measured for other doses in phase I. There was no prespecified enrollment in phase I. In phase II, additional patients were enrolled at the RP2D identified in phase I until a total planned sample of 26 patients was exposed to the RP2D. There was no planned minimum number of patients for either ALL or AML. The safety analysis set included all patients exposed to any dose of bendamustine, whereas the pharmacokinetic analysis set included all patients who received study drug and who had valid pharmacokinetic data. For the primary efficacy measure (ORR), a 1-sided 95% confidence interval was calculated based on the binomial distribution. If the lower boundary of this confidence interval was >5%, the null hypothesis (no worthwhile effect of bendamustine) of response rate of ≤5% was rejected. Summary statistics were provided only for the observed data; missing data were not imputed. Median DOR was assessed using the Kaplan-Meier method.
RESULTS
Patient Disposition and Characteristics
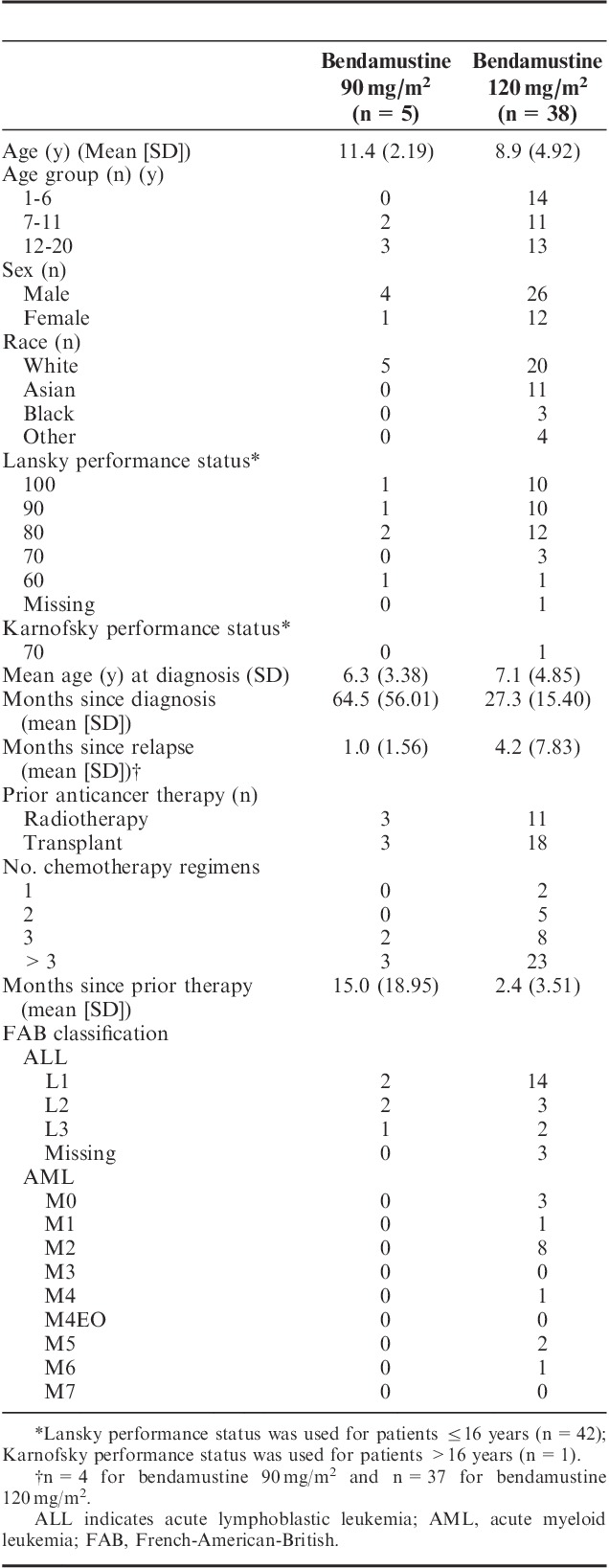
From August 2010 to July 2011, 11 patients were treated in the dose-escalation (phase I) portion of the study and 32 patients were treated in the efficacy and tolerability (phase II) portion (Fig. 2). The 2 phase I cohorts were expanded to replace individuals who were nonevaluable because of early disease progression. Overall, 27 patients with ALL and 16 patients with AML were enrolled from 24 centers. All patients had received multiple prior therapies, including 26 patients with >3 chemotherapy regimens and 21 patients with prior HSCT (Table 1). All 43 patients were eligible for efficacy and tolerability analyses.
FIGURE 2.
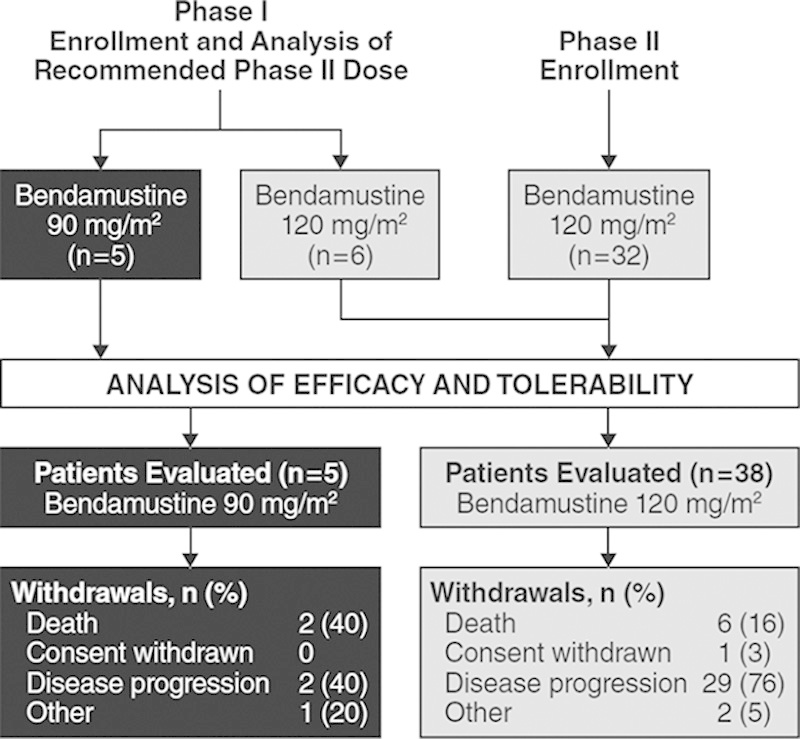
Patient disposition.
TABLE 1.
Patient Demographics and Baseline Clinical Characteristics
RP2D
In phase I, 5 patients received bendamustine 90 mg/m2 and 6 received bendamustine 120 mg/m2. No DLTs were observed with either dose. Among the 6 patients receiving 120 mg/m2, Cmax ranged from 3494 to 9137 ng/mL, AUC0-24 from 5322 to 14,039 ng h/mL, and AUC0-t from 5322 to 14,039 ng h/mL. Analysis of pharmacokinetic data from patients during phase I, along with a review of available preliminary safety and efficacy data, showed that the plasma concentrations attained from these pediatric patients were within the therapeutic range previously determined for adults.8 For this reason, escalation to the 150 mg/m2 dose level did not occur during phase I per protocol, and 120 mg/m2 was determined to be the RP2D. In phase II, all 32 patients received bendamustine 120 mg/m2. Overall, a total of 38 patients received the RP2D of bendamustine 120 mg/m2.
Study Drug Exposure
Among the 5 patients treated with bendamustine 90 mg/m2, 3 received 1 cycle, 1 received 2 cycles, and 1 received 8 cycles; the median total dose received was 182.0 mg/m2 (range, 177.0 to 1436.0 mg/m2). Among the 38 patients treated with bendamustine 120 mg/m2, 31 received 1 cycle and 7 received 2 cycles; the median total dose received was 241.5 mg/m2 (range, 233.0 to 498.0 mg/m2). Overall median dose intensity was 98.5% in the 90 mg/m2 group (range, 77.2% to 101%) and 99.8% in the 120 mg/m2 group (range, 56% to 123%).
Efficacy
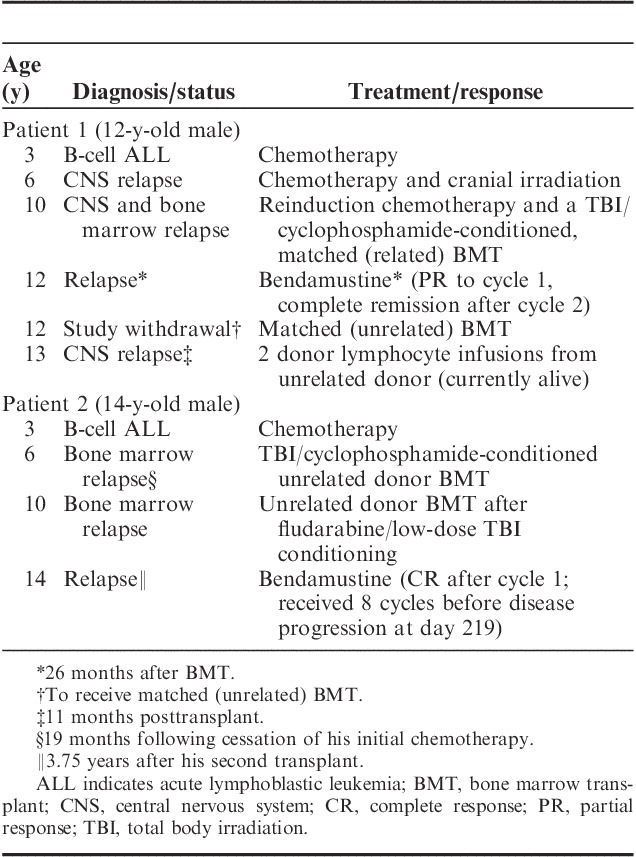
In phase I, 2 patients in the bendamustine 90 mg/m2 group (both with ALL) experienced a CR. Among the 38 patients who received bendamustine 120 mg/m2 in either phase, no patients experienced a CR or CRp (Table 2); thus, the primary efficacy measure was not achieved. In this group, 2 (5%) patients experienced a PR (both with ALL) and 7 (18%) had stable disease (4 with ALL and 3 with AML). The median DOR was not calculated for patients receiving 120 mg/m2 because of lack of complete responders; among patients in the phase I 90 mg/m2 dose group, 2 achieved CR, with 1 patient still in remission at last follow-up after unrelated HSCT. The details of the 2 patients who achieved a CR are outlined in Table 3.
TABLE 2.
Summary of Responses
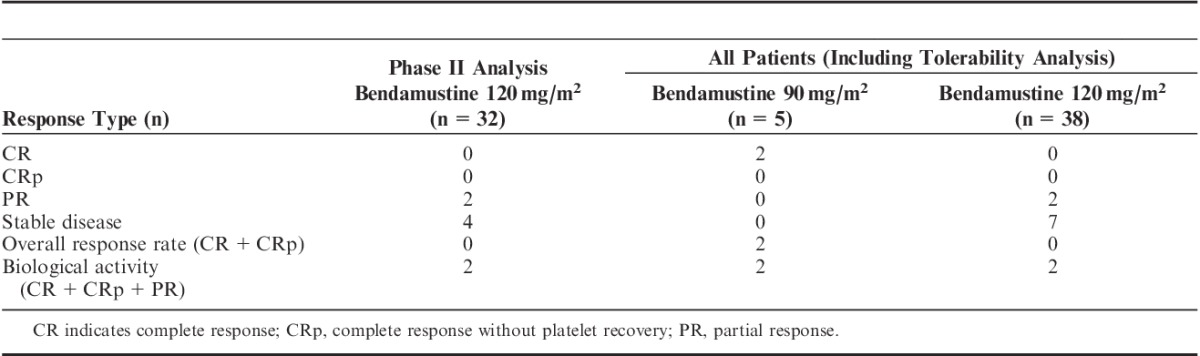
TABLE 3.
Efficacy Results of Patients Who Achieved CR Following Bendamustine 90 mg/m2
Tolerability
In these heavily pretreated patients, among those who received bendamustine 120 mg/m2, 3 patients experienced a dose delay beyond the standard 21-day cycle, because of thrombocytopenia (second cycle delayed until study day 43), febrile neutropenia (second cycle delayed until study day 39), and multiple skin rash (second dose of second cycle delayed 7 days) in 1 patient each. In addition, 1 patient in the 90 mg/m2 group experienced a dose delay (second cycle delayed until study day 30) because of tenderness at the port site. Doses could be delayed up to 2 weeks (for a maximum cycle length of 35 days) if a patient’s hematologic values did not recover and platelet counts could be supported by transfusion. In the second and final cycle for 1 of these patients, the dose was also reduced (to 90 mg/m2, per protocol) because of thrombocytopenia. Overall, the median length of cycle 1 was 21 days in both groups (90 mg/m2, n=5; 120 mg/m2, n=38), whereas cycle 2 was a median of 23.5 days in the 90 mg/m2 group (n=2) and 21 days in the 120 mg/m2 group (n=7).
Two deaths due to progressive disease occurred among patients receiving 90 mg/m2, with 1 death occurring ≤30 days (T-cell precursor acute leukemia) after the last dose and 1 death occurring >30 days after the last dose (B-ALL). Neither was associated with treatment. Among the 120 mg/m2 patients, 15 deaths occurred (14 from progressive disease and 1 with pancytopenia who died at home after further active medical treatment was declined); 6 occurred ≤30 days after the last dose; and 9 occurred >30 days after the last dose. None of the deaths were considered to be associated with treatment.
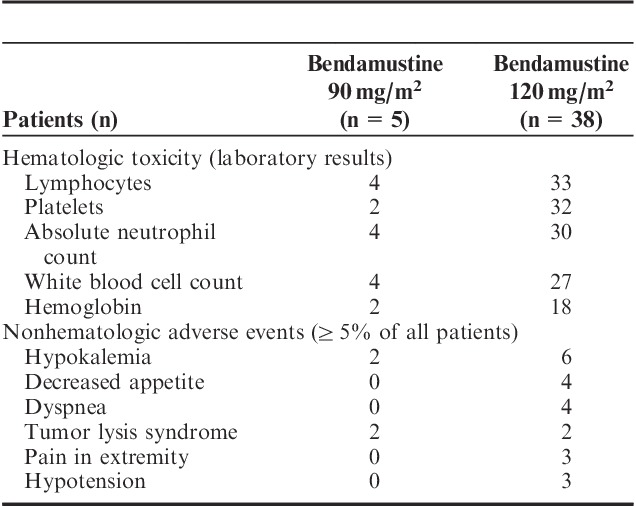
Overall, 33 patients (77%) experienced at least 1 serious adverse event; the most common serious adverse events were febrile neutropenia and infection. Among all 43 patients in the tolerability analysis, the most common adverse events (all grades) were anemia (65%), pyrexia (49%), nausea (47%), febrile neutropenia (35%), vomiting (35%), diarrhea (33%), and thrombocytopenia (33%). The most common grade 3/4 hematologic toxicities were decreased lymphocytes, platelets, and absolute neutrophil counts (Table 4). Forty-two patients experienced at least 1 patient-reported or investigator-reported nonhematologic grade 3 to 5 adverse event (any cause), and hypokalemia was the most common grade 3/4 adverse event (Table 4); 2 of the cases were considered related to treatment, both in the 12- to 20-year age group. One patient who received 120 mg/m2 withdrew from the study because of progressive disease (AML); based on study definitions, this qualified as significant worsening (change in nature, severity, or frequency) of the disease under study and was considered an adverse event.
TABLE 4.
Grade 3/4 Events
Pharmacokinetic Data Results
Among the entire cohort of patients receiving bendamustine at either 90 or 120 mg/m2, median tmax for the parent drug and its active metabolites was 1.1 hours, which was just after the end of the 1-hour infusion. Systemic exposure for all patients receiving bendamustine 120 mg/m2 was similar to that of the phase 1 group, with a mean Cmax of 7490 ng/mL and mean AUC0-t was 13,208 ng h/mL—again, the results were consistent with those reported for adults with indolent NHL8 (see Supplementary Table, Supplemental Digital Content 1, http://links.lww.com/JPHO/A56, for a summary of the pharmacokinetic results for bendamustine and its metabolites in pediatric patients).
DISCUSSION
Bendamustine has potential utility in relapsed or refractory lymphoid malignancies because of its lack of cross-reactivity with other alkylating agents. In vitro data suggest bendamustine to be more active in lymphoid versus myeloid disease (data on file, Teva Branded Pharmaceutical Products R&D Inc.). In general, relapsed ALL cells acquire resistance to all or most of the chemotherapeutic agents to which they are exposed.10 However, as demonstrated in the NCI-60 cell-line panel, bendamustine demonstrates limited cross-resistance with other alkylating agents (data on file, Teva Branded Pharmaceutical Products R&D Inc.). This has been borne out in clinical studies on adults, in which bendamustine showed superior activity compared with chlorambucil in patients with chronic lymphocytic leukemia4 and significant clinical activity despite prior alkylating therapy in patients with NHL.5 Thus, there is a theoretical basis for investigating whether bendamustine might have a role in the treatment of relapsed ALL or leukemia that is resistant to other chemotherapeutic agents, including alkylating agents, or as part of up-front therapy for patients who demonstrate resistant disease, such as those with minimal residual disease after induction therapy.
In vitro analysis of bendamustine in the AML cell line HL-60 indicated modest cytotoxicity6; however, hematologic response was not achieved in a pilot study of bendamustine in adult patients (median age, 69 y) with high-risk AML.11 Although this is a different AML population, these results are consistent with the lack of bendamustine activity observed in childhood AML patients in the present study. The lack of efficacy in this trial may limit future use of bendamustine monotherapy in heavily pretreated AML. However, bendamustine activity cannot be completely ruled out in untreated AML or possibly in conjunction with other therapy or at higher doses.
There are few data on the utility of bendamustine in the pediatric population. Indeed, to the best of our knowledge, this is the first study to demonstrate bendamustine activity in childhood relapsed or refractory ALL. The results of the present study suggest that bendamustine has an acceptable tolerability profile in pediatric patients with multiple relapsed or refractory ALL or AML. The most common serious grade ≥3 adverse event in this pediatric population with acute leukemia was hypokalemia, which is less common among adults with indolent lymphoma, but overall adverse events were similar to those reported for adult patients with indolent NHL.5,12 The RP2D was established as 120 mg/m2, which is identical to the single-agent dose that is used to treat adults with rituximab-refractory NHL. In addition, the pharmacokinetic results were consistent with those obtained from adult patients with indolent NHL.8 As these findings suggested that bendamustine dosed at 120 mg/m2 resulted in therapeutic bendamustine plasma levels, no patients were escalated to 150 mg/m2, as per protocol. The decision to rely on plasma concentration levels shown to be therapeutic in older patients with a different disease but yet hematologic, and thereby avoid subjecting patients in this study to potentially serious DLTs by increasing the dose, may have resulted in failure to administer a clinically effective dose of bendamustine in this patient population. Alternatively, it is not known if this heavily pretreated population including transplantation before the trial might have had a bone marrow reserve to recover adequately after a dose toxicity. A benefit-risk ratio assessment was carried out as well to move forward to RP2D based on current safety and pharmacokinetic results.
In this study, the primary efficacy measure was not achieved, as only 2 patients achieved an overall response. Interestingly, both patients were in the group receiving 90 mg/m2, which was not the RP2D. Furthermore, both responders had ALL; no activity was demonstrated in patients with AML. This is not entirely surprising given the in vitro data suggesting that bendamustine is more active in lymphoid rather than myeloid malignancies. However, the limited activity of bendamustine observed in this study is not unexpected for a single agent in such a very heavily pretreated population. As with other agents, bendamustine may result in a higher response rate when it is combined with other agents; thus, it should be further explored in combination regimens in pediatric patients with acute leukemias including ALL.13,14
Overall, the response data for this study population suggest that bendamustine is tolerable and has some activity in heavily pretreated patients with relapsed or refractory ALL but not in relapsed or refractory AML. Further studies are required to evaluate the role of bendamustine in combination with regimens that are the backbone of current leukemia therapy in children.
ACKNOWLEDGMENTS
The authors thank the sites and staff and patients and families who helped with and participated in the study. They also thank Philmore Robertson Jr., PhD, and Mary Bond, MS, MBA, from Teva Branded Pharmaceutical Products R&D Inc., who assisted with the pharmacokinetic schedule and data interpretation.
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.jpho-online.com.
Presented as a poster at the 53rd Annual Meeting of the American Society of Hematology, San Diego, CA, December 10–13, 2011.
This research was sponsored and conducted by Teva Branded Pharmaceutical Products R&D Inc., Frazer, PA. Funding for editorial, design, and production support was provided by Teva Branded Pharmaceutical Products R&D Inc., to The Curry Rockefeller Group LLC, Tarrytown, NY. C.F. received funding from Teva to present this work at ASH in 2011.
L.F. is employed by PPD-PharmacoVigilance (contractual relationship with Teva Branded Pharmaceutical Products R&D Inc.). D.B.K., M.M., and J.W. were employed by Teva Branded Pharmaceutical Products R&D Inc. at the time the study was conducted. The remaining authors declare no conflict of interest.
REFERENCES
- 1.Bhatia U, Danishefsky K, Traganos F, et al. Induction of apoptosis and cell cycle-specific change in expression of p53 in normal lymphocytes and MOLT-4 leukemic cells by nitrogen mustard.Clin Cancer Res. 1995;1:873–880 [PubMed] [Google Scholar]
- 2.Strumberg D, Harstrick A, Doll K, et al. Bendamustine hydrochloride activity against doxorubicin-resistant human breast carcinoma cell lines.Anticancer Drugs. 1996;7:415–421 [DOI] [PubMed] [Google Scholar]
- 3.Leoni LM, Bailey B, Reifert J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents.Clin Cancer Res. 2008;14:309–317 [DOI] [PubMed] [Google Scholar]
- 4.Knauf WU, Lissichkov T, Aldaoud A, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia.J Clin Oncol. 2009;27:4378–4384 [DOI] [PubMed] [Google Scholar]
- 5.Friedberg JW, Cohen P, Chen L, et al. Bendamustine in patients with rituximab-refractory indolent and transformed non-Hodgkin’s lymphoma: results from a phase II multicenter, single-agent study.J Clin Oncol. 2008;26:204–210 [DOI] [PubMed] [Google Scholar]
- 6.Konstantinov SM, Kostovski A, Topashka-Ancheva M, et al. Cytotoxic efficacy of bendamustine in human leukemia and breast cancer cell lines.J Cancer Res Clin Oncol. 2002;128:271–278 [DOI] [PubMed] [Google Scholar]
- 7.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia.J Clin Oncol. 2003;21:4642–4649 [DOI] [PubMed] [Google Scholar]
- 8.Owen JS, Melhem M, Passarell JA, et al. Bendamustine pharmacokinetic profile and exposure-response relationships in patients with indolent non-Hodgkin’s lymphoma.Cancer Chemother Pharmacol. 2010;66:1039–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institutes of Health Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. NIH Publication No. 09-5410. 2009Bethesda, MD:National Institutes of Health, Department of Health and Human Services [Google Scholar]
- 10.Klumper E, Pieters R, Veerman AJ, et al. In vitro cellular drug resistance in children with relapsed/refractory acute lymphoblastic leukemia.Blood. 1995;86:3861–3868 [PubMed] [Google Scholar]
- 11.Strupp C, Knipp S, Hartmann J, et al. A pilot study of bendamustine in elderly patients with high-risk MDS and AML.Leuk Lymphoma. 2007;48:1161–1166 [DOI] [PubMed] [Google Scholar]
- 12.Treanda [package insert] 2012Frazer, PA:Teva Pharmaceutical Industries Ltd [Google Scholar]
- 13.Fowler N, Kahl BS, Lee P, et al. Bortezomib, bendamustine, and rituximab in patients with relapsed or refractory follicular lymphoma: the phase II VERTICAL study.J Clin Oncol. 2011;29:3389–3395 [DOI] [PubMed] [Google Scholar]
- 14.Weide R, Hess G, Köppler H, et al. High anti-lymphoma activity of bendamustine/mitoxantrone/rituximab in rituximab pretreated relapsed or refractory indolent lymphomas and mantle cell lymphomas. A multicenter phase II study of the German Low Grade Lymphoma Study Group (GLSG).Leuk Lymphoma. 2007;48:1299–1306 [DOI] [PubMed] [Google Scholar]


