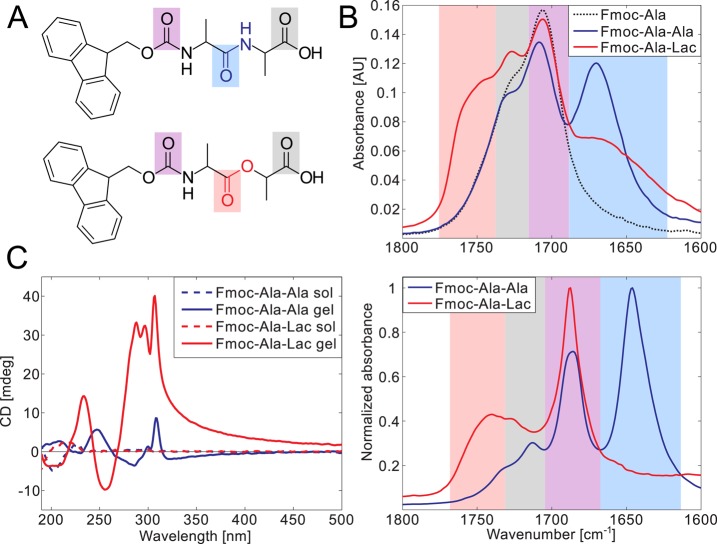
Figure 2.
Fmoc-Ala-Ala and Fmoc-Ala-Lac are spectrally distinct in both their gelled and nongelled forms. Carbonyl bonds are highlighted in the chemical structures of Fmoc-Ala-Ala (A, top) and Fmoc-Ala-Lac (A, bottom), and the resonance stretching frequencies of these particular bonds are highlighted in the same color in the IR spectra of solutions in ethanol (B, top) and gels in water (B, bottom). The spectral overlap can be correlated with carbonyl features, with the carbamate linker’s carbonyl stretching (purple) and the terminal carboxylic acid carbonyl (gray) common to Fmoc-Ala-Ala, Fmoc-Ala-Lac, and Fmoc-Ala (structure not shown). CD spectra (C) show a marked increase in dichroism in the gelled state (solid lines) relative to that in the solution state (high pH, dashed lines) in both Fmoc-Ala-Ala and Fmoc-Ala-Lac, indicating induced chirality as a result of self-assembly. Large peaks above 280 nm indicate the interaction of aromatic Fmoc groups within a chiral assembly.