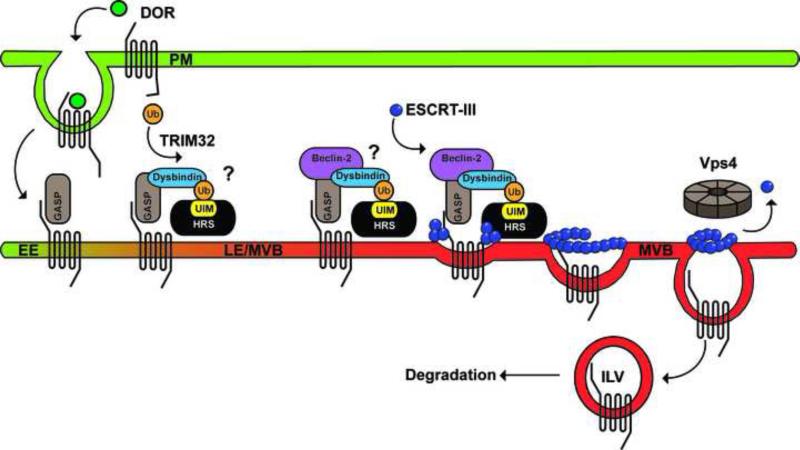
Figure 2. GASP-1 mediated lysosomal sorting of DOR.
Upon ligand stimulation, DOR is internalized from the plasma membrane (PM) to early endosomes (EE), where GASP-1 binds directly to the C-tail domain of DOR and facilitates lysosomal degradation via ESCRT-III and Vps4. Dysbindin also binds to GASP-1 and HRS, a component of ESCRT-0, and mediates lysosomal degradation of DOR. A recent study now indicates that Beclin-2, a component of the autophagy machinery, has a distinct function in lysosomal degradation of GPCRs including DOR that requires interaction with GASP-1. Although ubiquitination of DOR is not required for lysosomal degradation, it is possible that ubiquitination of dysbindin mediated by the RING-domain E3 ligase TRIM32 or some other component of this complex mediates interaction with HRS via its ubiquitin-interacting motif (UIM), but this has not been tested. In addition, the order and timing of GASP-1, Beclin-2 and HRS recruitment to DOR remains unclear.