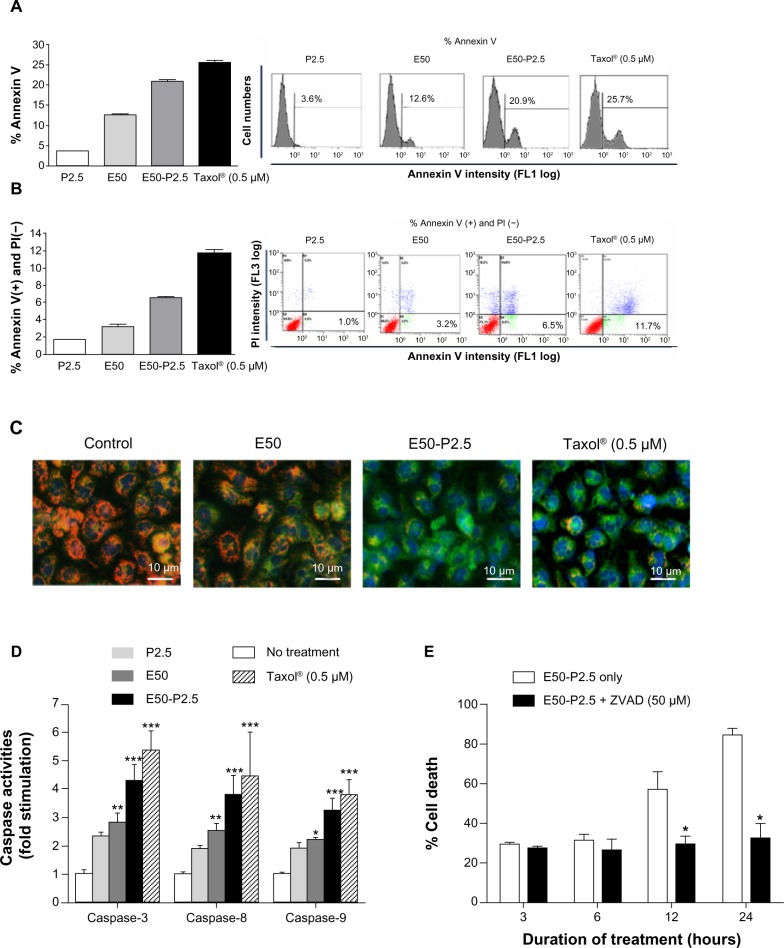
Figure 3.
Enhanced apoptosis induction of EGCG by pNG in B16F10 cells.
Notes: (A) The fractions of annexin V-positive B16F10 cells were 3.59%±0.30%, 12.63%±0.61%, 20.93%±0.55%, and 25.7%±0.54%, after treatment with pNG 2.5 ppm, EGCG 50 μM, EGCG-pNG 50 μM:2.5 ppm, and Taxol® (Sigma-Aldrich; St Louis, MO, USA) 0.5 μM, respectively, at 24 hours; (B) the fractions of annexin V-positive and PI-negative B16F10 cells were 1.0% ± 0.17%, 3.2% ± 0.23%, 6.5% ± 0.23%, and 11.7% ± 0.41%, after treatment with pNG 2.5 ppm, EGCG 50 μM, EGCG-pNG 50 μM:2.5 ppm, and Taxol® 0.5 μM, respectively, at 24 hours; (C) cells treated with EGCG-pNG for 24 hours expressed more green fluorescence than those treated with EGCG (magnification ×200). Depolarized mitochondria are indicated by green fluorescence (JC-10 monomer), and polarized mitochondria are indicated by orange fluorescence (aggregated JC-10). Cell nuclei are indicated by blue fluorescence coupled with Hoechst 33342 staining. Scale bar, 10 μm; (D) cells treated with EGCG-pNG for 24 hours showed a significant increase of caspase-3, -8, and -9 activity compared with those treated with EGCG; (E) initiation of time-dependent apoptotic activation by EGCG-pNG treatment via the caspase pathway in B16F10 melanoma cells. Data shown are mean ± standard deviation for three samples. Data containing asterisks are significantly different from the control values at *P<0.05; **P<0.01; ***P<0.001.
Abbreviations: PI, propidium iodide; EGCG, (–)-epigallocatechin-3-gallate; pNG, physical nanogold; P2.5, pNG 2.5 ppm; E50, EGCG 50 μM; E50-P2.5, EGCG-pNG 50 μM:2.5 ppm; ZVAD, Z-VAD-FMK, N-Benzyloxycarbonyl-Val-Ala-Asp (O-Me) fluoromethyl ketone.