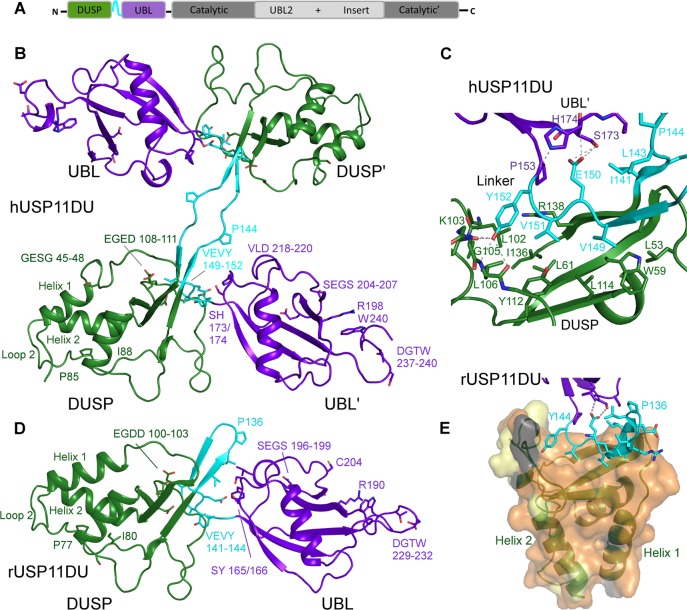
Figure 1.
Structure of USP11 N-terminal DUSP-UBL domains. (A) Schematic representation of the USP11 domain structure. (B) Cartoon representation depicting human USP11DU in a dimeric domain swapped arrangement as observed in the crystallographic asymmetric unit. The second copy is labeled as ′. The DUSP domains from each chain are shown in green, the UBL domains are in purple, and the linker region, residues 141–152, is shown in cyan. (C) Close-up view of the DUSP–linker interaction in hUSP11. Note the VEVY motif, part of the linker region shown in cyan and the hydrophobic nature of side chains in the DUSP domain, shown in green. H-bonding interactions are indicated as dashed lines. (D) Cartoon depicting monomeric rat USP11DU in the same orientation as hUSP11DU in (B). The DUSP domain depicted in green stacks against the UBL domain from the same chain, shown in purple. This arrangement is mediated by the linker region, residues 133–144, which forms a β-hairpin structure denoted the DU finger. Key residues and sequence segments are labeled. (E) Cartoon depicting the hydrophobic DUSP-linker interface. The rUSP11 DUSP surface is colored according to sequence conservation between USP11 from human and rat. Identical residues are colored orange, residues that display similar properties are shown in yellow, and residues that are weakly similar in light gray and dissimilar in dark gray.