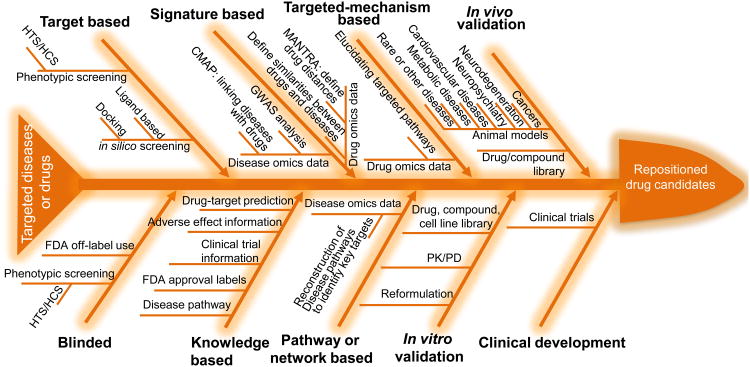
Figure 2.
Fishbone flowchart of drug-repositioning pipelines. Developed drug-repositioning pipelines comprise at least one of these methods (i.e., blinded, target based, knowledge based, signature based, pathway or network based, and targeted-mechanism based), preclinical studies (in vitro and/or in vivo validations) and clinical development (testing the new indications identified from the preclinical development). Development of a new drug-repositioning pipeline for a drug or a disease of interest should evaluate the priorities of these repositioning methods based on the available information of the drug or the disease. For example, an infectious disease that only has limited available signaling information related to cell wall and cytoplasmic membrane proteins would lead to high priority of target-based drug-repositioning studies focusing on these cell wall and cytoplasmic membrane proteins. Many drug-repositioning pipelines can reverse the order of the listed methods in the fishbone flowchart and make use of them flexibly. As an example, several existing drug-repositioning pipelines first consider pathway- or network-based drug-repositioning methods to reconstruct disease pathways and then use knowledge-based or targeted-based methods to identify candidate drugs. The fishbone provides all relevant components of general drug-repositioning pipelines, and one can customize specific drug-repositioning pipelines according to the available knowledge and information of targeted drugs or diseases. Abbreviations: FDA, Food and Drug Administration; GWAS, genome-wide association study; HTS/HCS, high-throughput and/or high-content screening; PK/PD, pharmacokinetics/pharmacodynamics.