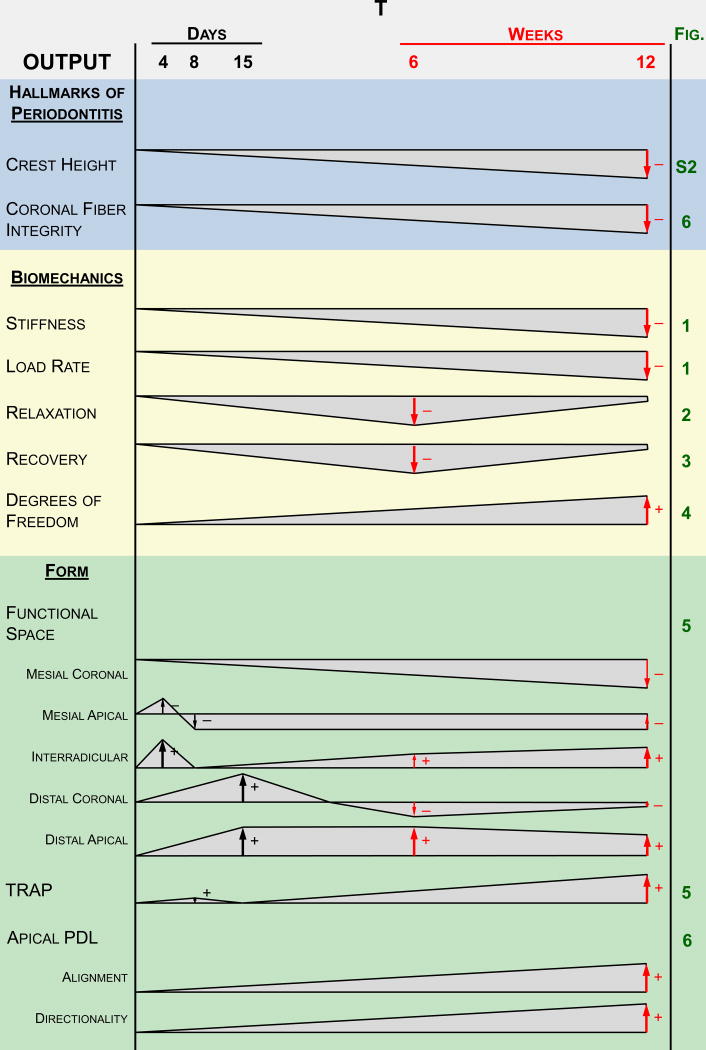
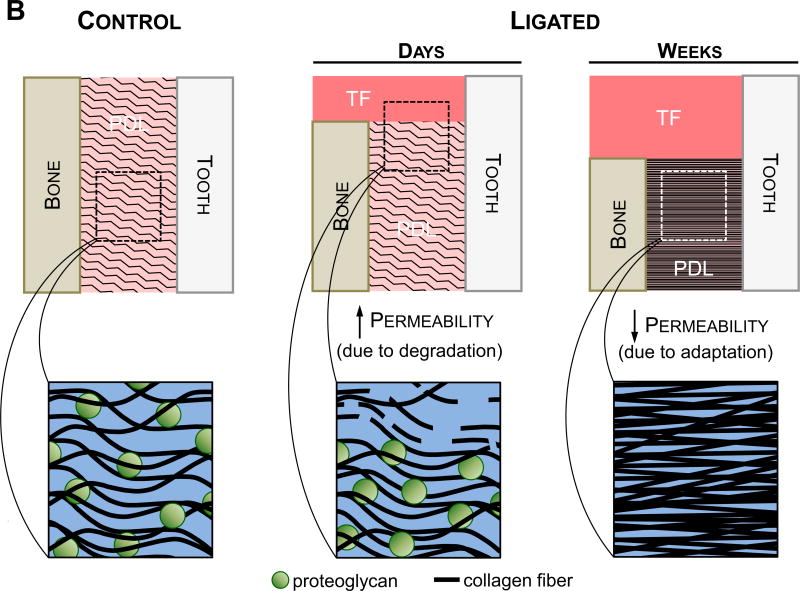
Figure 7. Suggested model summarizing the findings in a temporal fashion.
(A) These observations are based on the hallmarks of periodontitis, joint biomechanics, and morphological features of the soft tissue and the overall joint. Polygonal shapes illustrate deviation of the diseased joint from the control joint based on magnitude. The negligible differences at the beginning of the timeline represent no differences in baseline measurements between the two groups at the start of ligation. Arrows represent the increase and/or decrease in deviation. (B) Illustrations showing the suggested differences in permeability that correlate to the findings at both the early stages (4-15 days) (Lee and Lin et al., 2013) and later stages (6-12 weeks) of the study. Permeability to fluid flux is expected to increase during the initial degradative phase of the disease, during which the host response to inflammation causes degeneration of transseptal fibers and a decrease in crestal bone height. Strain-induced adaptation due to the increased mobility of the tooth is observed within the apical regions of the PDL at later stages of periodontitis, leading to increased fibrous tissue formation and a decrease in proteoglycan content. Permeability of the tissue is decreased leading to a decrease in load relaxation and load recovery characteristics in the diseased fibrous joint at a later stage. As such, the cause of decreased stiffness and load rate is attributed to a loss in PDL attachment and degradation of the coronal aspects of the joint, while the decrease in load relaxation (at higher loads) and recoverability (at lower loads) can be attributed to adaptation of the joint. Please note that the observed biomechanically-induced adaptations outlined in this model are reserved to the soft tissue elements of the fibrous joint.