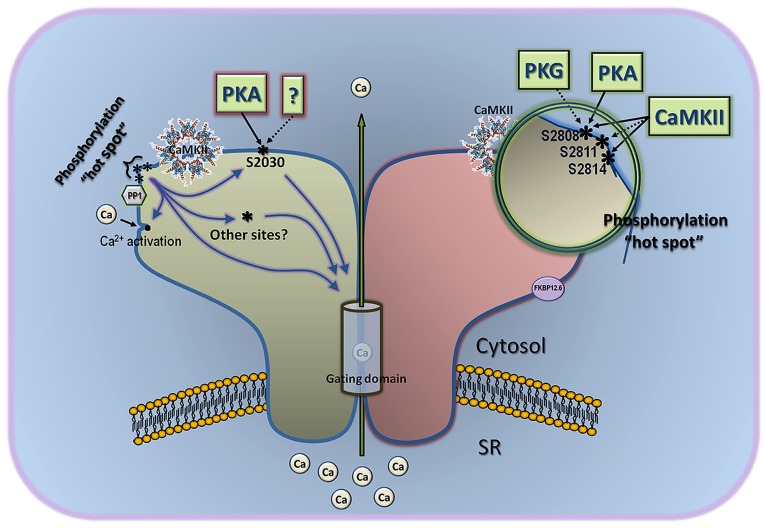
FIGURE 1.
Multi-site model of RyR2 phosphorylation. This model considers the three phospho-sites known to date, and gives also significant weight to other as-yet-uncovered sites. The classical sites S2808 and S2814 are part of a “phosphorylation hotspot” that is located in a protruding part of the channel, is targeted by several kinases, and may contain other phospho-epitopes not yet characterized (for example, S2811). Phosphorylation of individual residues within this hotspot may be undistinguishable by the channel’s gating domain, but gradual addition of phosphate groups here may contribute to a tunable effect instead of an all-or-none response. Assuming that the phosphorylation hotspot works collectively toward a single effect, the differential regulation of PKA and CaMKII on channel gating may come about by the combined effect of each kinase on phospho-residues of the hotspot and other phosphorylation sites, such as S2030. This model also accommodates solo effects of S2030 or the phosphorylation hotspot on gating domains of the channel, as well as indirect effect on gating via interaction with classical Ca2+ activation sites. CaMKII and protein phosphatase 1 (PP1) are depicted close to the phosphorylation hotspot because the latter is readily phosphorylated/dephosphorylated by endogenous CaMKII and PP1. Demonstrated effect of kinases on S2808, S2814, and S2030 in intact cells or hearts is shown with a solid line, and in vitro effect is shown with a broken line.