Abstract
Purpose
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is characterized by prolonged clinical symptoms even after the withdrawal of the culprit drug. Different criteria to diagnose DRESS syndrome have been proposed; however, there have been limited studies on prognostic factors. We investigated appropriate criteria for the diagnosis of DRESS syndrome in practice and with associated prognostic factors.
Methods
A total of 48 patients with DRESS syndrome that satisfied RegiSCAR possible (or more) criteria were retrospectively recruited. They were also analyzed according to Bocquet's criteria and Japanese drug-induced hypersensitivity syndrome (DIHS) criteria. The duration of clinical manifestations, requirement for steroids, and fatalities determined the severity of DRESS syndrome. Blood tests were performed at initial presentation to our hospital.
Results
A total of 60.4% of patients satisfied RegiSCAR definite criteria and 77.1% satisfied Bocquet's criteria. Only 18.8% satisfied atypical DIHS criteria from the Japanese group. A total of 96.6% patients who fit the RegiSCAR definite criteria, 96.6% also satisfied Bocquet's criteria; reciprocally, 75.7% of patients who met Bocquet's criteria also satisfied RegiSCAR definite criteria. The duration of clinical symptoms positively correlated with leukocyte, lymphocyte, and eosinophil counts in non-fatal cases. Lymphocyte counts were higher in patients who used steroids compared to steroid-naïve patients. Fatal cases showed higher serum creatinine and ferritin levels compared to non-fatal cases.
Conclusions
Bocquet's criteria is efficient and appropriate to diagnose DRESS syndrome in clinical practice. Lymphocyte and eosinophil counts as well as creatinine and ferritin levels could be useful early prognostic factors.
Keywords: Diagnosis, drug hypersensitivity, lymphocytes, prognosis
INTRODUCTION
DRESS syndrome is characterized by fever, cutaneous eruption, internal organ involvement, and hematologic abnormalities 1-8 weeks after the administration of the culprit drug,1 with an incidence ranging from 1 in 1,000 to 1 in 10,000 exposures in patients taking the drug.2,3 In 1996, Bocquet et al.4 proposed the term DRESS syndrome and its diagnostic criteria. In 2006, Shiohara et al.5 proposed a set of criteria for the diagnosis of DIHS. In 2007, The European Registry of Severe Cutaneous Adverse Reactions to Drugs and Collection of Biological Samples (RegiSCAR) group suggested different diagnostic criteria for DRESS syndrome.6 These 3 different sets of criteria can impede a proper diagnosis and assessment of DRESS syndrome.7 However, no studies have conducted a comparative analysis between the different criteria.
Unlike other drug allergic diseases the clinical manifestations of DRESS syndrome prolong for several weeks even after stopping the culprit drug.4 DRESS syndrome is one of the most severe adverse drug reactions and may be life-threatening with mortality rates of about 10%.8 However, there is limited information on the prognostic factors for DRESS syndrome.3
We investigated which criteria are the most appropriate to diagnose DRESS syndrome in practice and what factors are important to predict the prognosis at the onset of DRESS syndrome.
MATERIALS AND METHODS
Patients
The present study retrospectively analyzed clinical data collected from patients who were admitted to Chonnam National University Hospital due to adverse drug reactions between January 2005 and July 2012. We enrolled patients that satisfied the RegiSCAR possible (or more) criteria for DRESS syndrome.6 Our hospital ethical review board approved this study.
Diagnostic criteria for DRESS syndrome
Three different sets of criteria were used to diagnose DRESS syndrome. RegiSCAR criteria include at least 3 of the following 7 characteristics: 1) skin eruption, 2) fever (>38℃), 3) lymphadenopathy at least 2 sites, 4) involvement of at least 1 internal organ, 5) lymphocytosis (>4×103/µL) or lymphocytopenia (<1.5×103/µL), 6) blood eosinophilia (>10% or 700/µL), and 7) thrombocytopenia (<120×103/µL). Patients were classified into definite, probable, possible or no cases according to the RegiSCAR scoring system.6
Bocquet's criteria require meeting the following 3 features: 1) skin eruption, 2) blood eosinophilia (>1.5×103/µL) or the presence of atypical lymphocytes, and 3) internal organ involvement, including lymphadenopathies (>2 cm in diameter), hepatitis (liver transaminases values > twice the upper normal limit), interstitial nephritis, and interstitial pneumonia or carditis.4
The criteria established by the Japanese group to diagnose DIHS include the following features: 1) maculopapular rash developing >3 weeks after starting a limited number of drugs, 2) prolonged clinical symptoms 2 weeks after discontinuing the causative drug, 3) fever (>38℃), 4) elevation of liver enzyme (alanine aminotransferase [ALT] >100 U/L) or involvement of other organs, 5) leukocytosis (>11×103/µL), atypical lymphocytosis (>5%) or eosinophilia (>1.5×103/µL), 6) lymphadenopathy, and 7) human herpesvirus (HHV)-6 reactivation. Diagnosis of typical DIHS requires the presence of all 7 criteria. Atypical DIHS is diagnosed in patients with 1)-5).9 Serum HHV-6 DNA was detected by polymerase chain reaction (2720 Thermal Cycler, Applied Biosystems, Singapore). Organ involvement was defined according to a study by Chen et al.10 Lymphadenopathies were determined by physical examination or computed tomography.
Blood tests
Complete blood count with differential, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), aspartate aminotransferase (AST), ALT, total bilirubin, creatinine, lactate dehydrogenase (LDH), and ferritin were measured. Autoimmune or infectious tests were performed to exclude the potential for underlying diseases, if indicated. The blood tests were performed at initial presentation to our hospital.
Causative drugs
Culprit drugs were assessed using Naranjo's scale11 and the World Health Organization-Uppsala Monitoring Center classification.12 Possible or more probable drugs were considered as culprits to induce DRESS syndrome.
DRESS syndrome severity
DRESS syndrome severity was assessed using the duration of clinical symptoms, requirement for systemic steroid treatment, or fatality. The duration of clinical manifestations was defined as the period from onset to the complete disappearance of clinical symptoms and normalization of laboratory abnormalities after treatment. Steroids were used when DRESS syndrome involves a severe or life-threatening condition of the skin and internal organs.2
Statistical analysis
Data are expressed as medians (ranges). Comparisons were performed with a Mann-Whitney U-test. Correlations were analyzed using Spearman's coefficients (Rs). A coefficient of agreement between the diagnostic criteria was analyzed by crossover analysis and the kappa coefficient (κ).13 The SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL, USA) program was used for all analyses. A P value of <0.05 was considered to indicate statistical significance.
RESULTS
Demographic data
A total of 48 patients (29 females) fulfilled the RegiSCAR possible (or more) criteria. A case reported by Wi et al.14 was included in the present study. Age ranged from 3 to 89 years. The underlying diseases were hypertension in 15 patients (31.3%), tuberculosis in 6 (12.5%), gout in 6 (12.5%), congestive heart failure in 3 (6.3%), schizophrenia in 3 (6.3%), seizure in 3 (6.3%), diabetes mellitus in 2 (4.2%), chronic renal failure in 2 (4.2%), solid tumors in 2 (4.2%), bipolar disorder in 1 (2.1%), chronic obstructive lung disease in 1 (2.1%), and hematologic malignancy in 1 (2.1%).
Comparisons between diagnostic criteria
A total of 37 patients (77.1%) satisfied Bocquet's criteria, 45 patients (93.3%) satisfied RegiSCAR probable (or more criteria), 29 patients (60.4%) satisfied RegiSCAR definite criteria, and 9 patients (18.8%) satisfied the atypical DIHS criteria of the Japanese group. Typical DIHS criteria could not be assessed in all cases, because tests for HHV-6 DNA were only performed in 20 cases; subsequently, HHV-6 DNA was detected in only one patient.
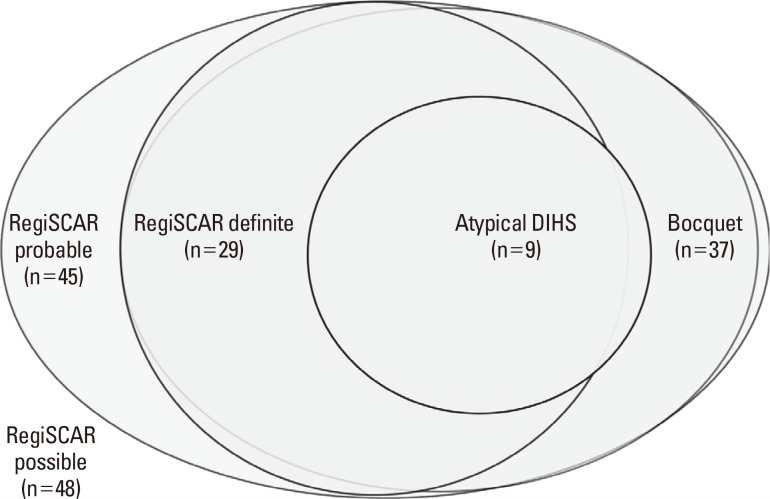
Among the 45 patients who met RegiSCAR probable (or more criteria), 36 (80.0%) satisfied Bocquet's criteria and 9 (20.0%) satisfied atypical DIHS criteria. Among 29 patients who met the RegiSCAR definite criteria, 28 (96.6%) satisfied Bocquet's criteria and 8 (27.6%) satisfied atypical DIHS criteria. A total of 9 patients that met atypical DIHS criteria satisfied RegiSCAR probable (or more) criteria in all and RegiSCAR definite criteria in 8 (88.9%) (Fig. 1).
Fig. 1.
Proportion of patients who satisfied different criteria for DRESS syndrome. RegiSCAR possible means the group of patients that satisfied possible RegiSCAR (or more) criteria in the scoring system; RegiSCAR probable means the group of patients that satisfied probable or more criteria; RegiSCAR definite means the group of patients that satisfied definite criteria; Bocquet means the group of patients that satisfied Bocquet's criteria; Atypical DIHS means the group of patients that satisfied atypical DIHS criteria.
Among the 37 patients that fulfilled Bocquet's criteria, 36 (97.3%) satisfied RegiSCAR probable (or more) criteria and 28 (75.7%) satisfied the RegiSCAR definite criteria. A total of 8 patients satisfied RegiSCAR probable criteria and 1 satisfied RegiSCAR possible criteria out of the 9 patients who fulfilled Bocquet's criteria but did not meet RegiSCAR definite criteria. Among 37 patients that met Bocquet's criteria, 9 (24.3%) satisfied atypical DIHS criteria. All 9 patients who fulfilled atypical DIHS criteria satisfied Bocquet's criteria (Fig. 1).
The coefficient of agreement between Bocquet's criteria and RegiSCAR definite criteria was 0.53, which indicated moderate agreement.15 The coefficients between Bocquet's criteria and atypical DIHS criteria and between RegiSCAR definite criteria and atypical DIHS criteria were 0.13 and 0.19, respectively.
Out of 11 patients who did not fulfill Bocquet's criteria, 3 were finally diagnosed with DRESS syndrome, 4 with drug fever, 2 with drug-induced liver disease, 1 with serum sickness-like reaction, and 1 with no adverse drug reaction. The 3 patients with DRESS syndrome satisfied RegiSCAR probable criteria.
Clinical characteristics
We analyzed the clinical features of 37 patients who satisfied Bocquet's criteria. Clinical symptoms began 21 days (range, 1-88) after the initial administration of culprit drugs and continued for 32 days (8-108) after the withdrawal of drugs. A total of 3 patients (8.3%) died; 2 due to pneumonia and 1 due to a intracerebral hemorrhage, which was supposedly related to DRESS syndrome (Table 1).
Table 1.
Clinical findings of the three fatal cases
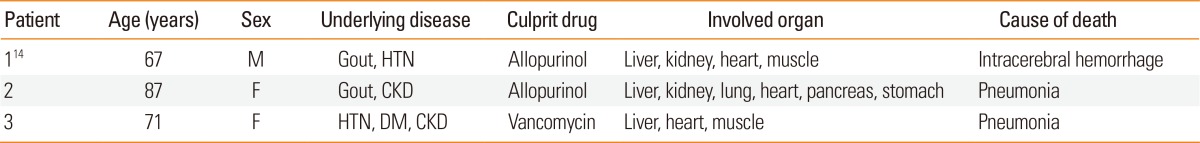
M, male; F, female; HTN, hypertension; CKD, chronic kidney disease; DM, diabetes mellitus.
Cutaneous symptoms were maculopapular rash in 34 patients (91.9%), facial edema in 6 (16.2%), peripheral edema in 4 (10.8%), bullae in 4 (10.8%), vesicles in 4 (10.8%), and pustules in 1 (2.7%). The involved organs were the liver in 30 patients (81.1%), kidney in 24 (64.9%), lung in 16 (43.2%), lymphadenopathies in 14 (37.8%), heart in 13 (35.1%), muscle in 5 (13.5%), pancreas in 4 (10.8%), and gall bladder in 2 (5.4%). Antibiotics (12 patients, 32.4%), including cephalosporin, vancomycin, meropenem, amoxicillin, and tazobactam, were the most common culprit drugs. Anticonvulsants (8 patients, 21.6%) including carbamazepine, valproic acid, and lamotrigine, allopurinol (7 patients, 18.9%), anti-tuberculosis drugs (4 patients, 10.8%) including isoniazid, and ethambutol, NSAIDs (2 patients, 5.4%), and dapsone (1 patient, 2.7%) were followed. Blood tests at initial presentation showed that values of white blood cells (WBC), eosinophils, CRP, AST, ALT, LDH, and ferritin were increased (Table 2). All patients stopped the culprit drugs. Systemic steroids and intravenous immunoglobulin (IVIG) were used in 23 (62.2%) and 5 patients (13.5%), respectively. Systemic steroids were administered for 18 days (range, 2-179). IVIG was infused for 5 days (range, 3-5).
Table 2.
Laboratory findings at initial presentation in 37 patients with DRESS syndrome who satisfied Bocquet's criteria
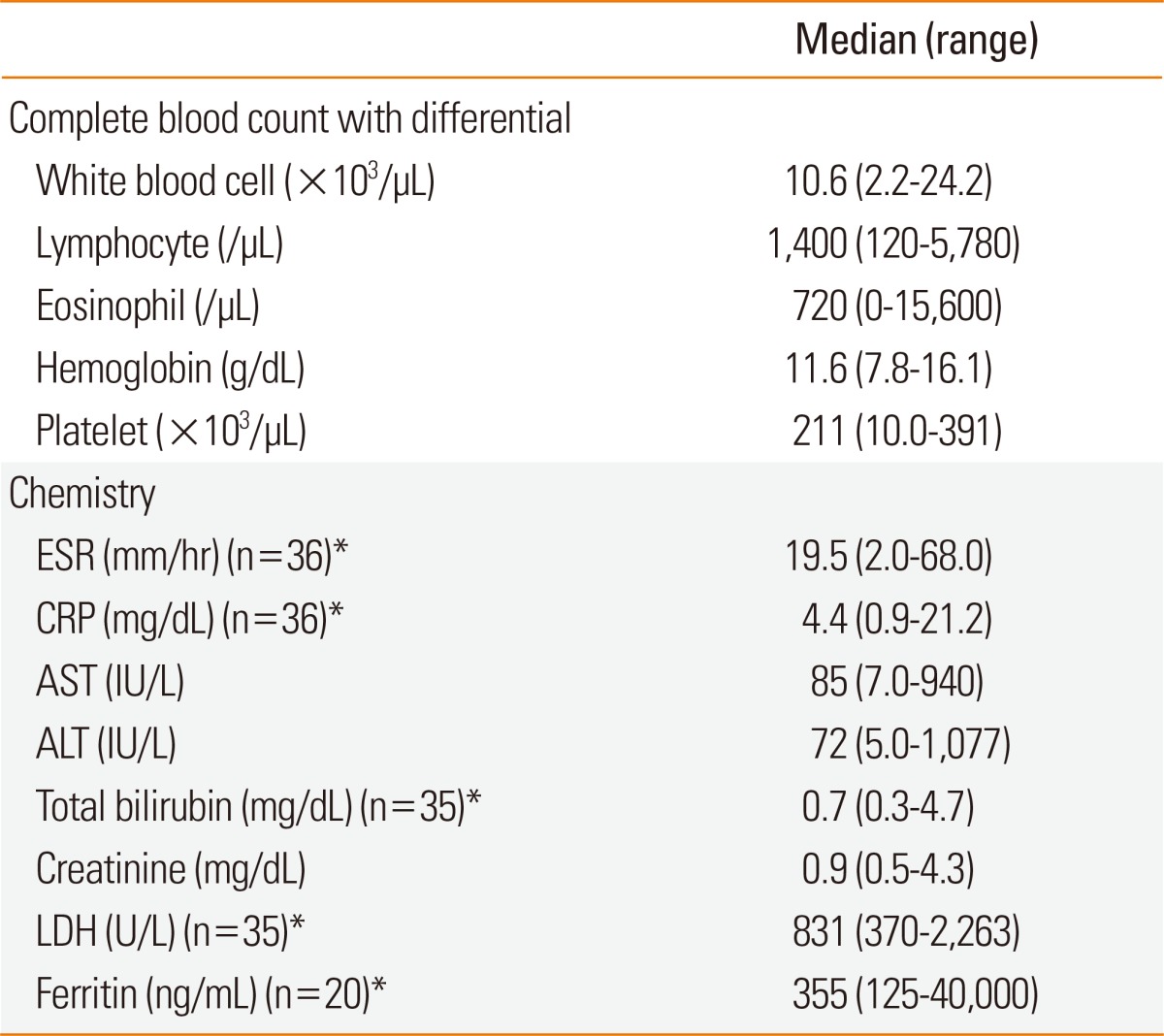
*The figure in parenthesis means the number of patients analyzed.
ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase.
Factors contributing to DRESS syndrome severity
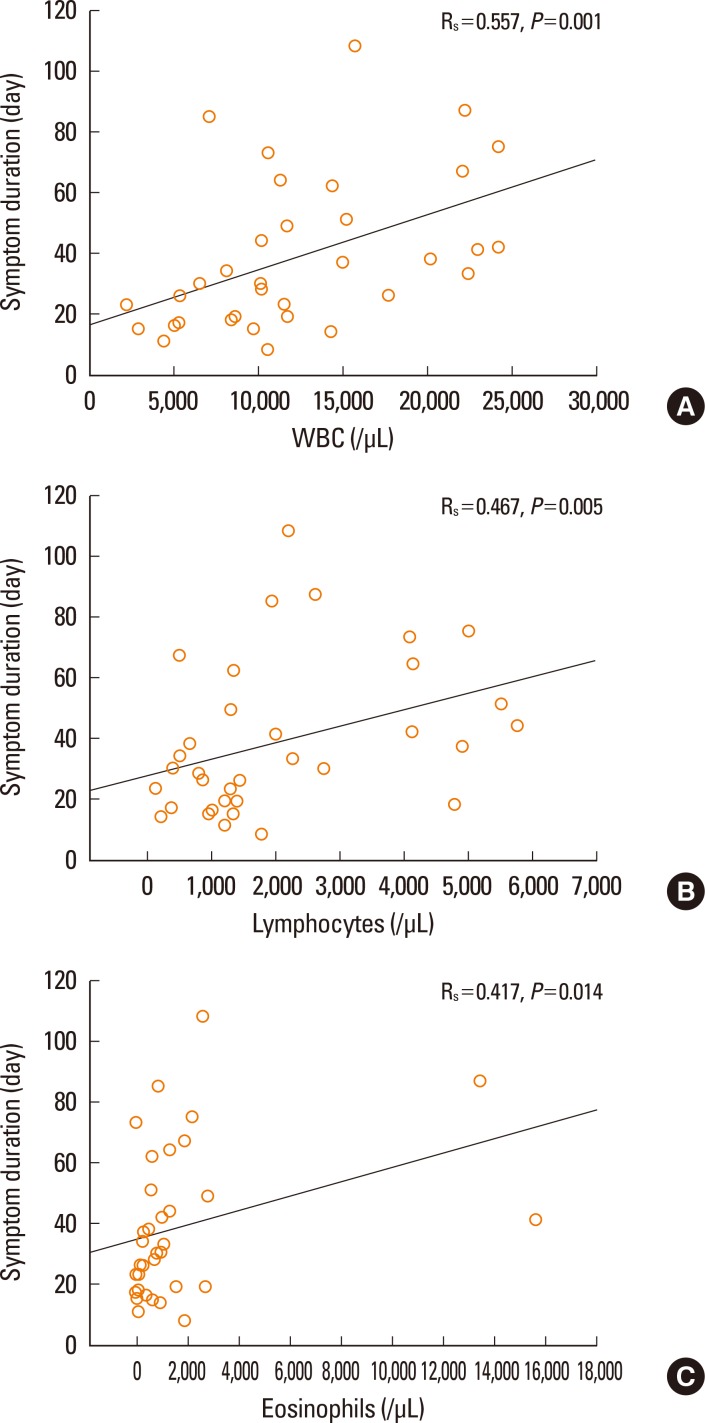
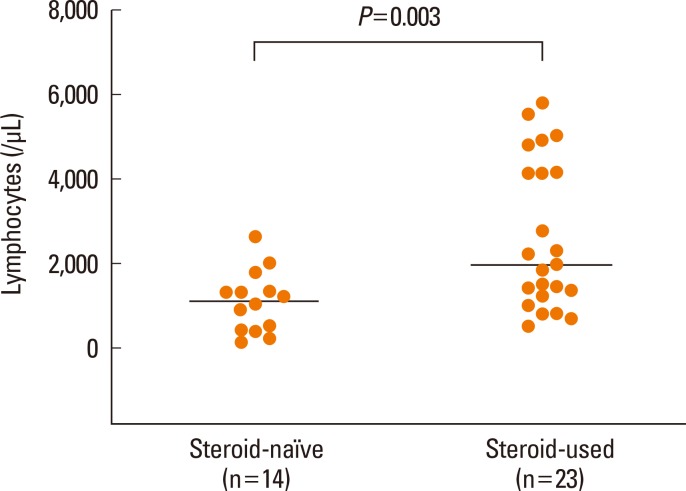
First, we analyzed factors contributing to clinical severity in 37 patients who satisfied Bocquet's criteria. The duration of clinical symptoms was correlated with WBC count (P=0.001, Fig. 2A), lymphocyte count (P=0.005, Fig. 2B), and eosinophil count (P=0.014, Fig. 2C), but not with hemoglobin, platelets, ESR, CRP, AST, ALT, total bilirubin, creatinine, LDH, or ferritin, in non-fatal cases. The duration of medication before symptoms was not related to the duration of clinical symptoms. The duration of clinical symptoms did not differ according to the culprit drug. Patients given systemic steroids showed higher blood lymphocyte counts compared to those not given steroids (P=0.003, Fig. 3). There were no differences in the values of WBC, eosinophil, hemoglobin, platelet, ESR, CRP, AST, ALT, total bilirubin, creatinine, LDH, ferritin, or the duration of medication before symptoms between steroid-used and steroid-naïve patients. Fatal cases showed higher serum creatinine (P=0.008, Fig. 4A) and ferritin (P=0.012, Fig. 4B) compared to non-fatal cases. There were no differences in the values of WBC, lymphocyte, eosinophil, hemoglobin, platelet, ESR, CRP, AST, ALT, total bilirubin, LDH, or the duration of medication before symptoms between fatal and non-fatal patients.
Fig. 2.
Correlations between blood (WBCs) (A), lymphocytes (B), or eosinophils (C) and the duration of clinical symptoms in 34 non-fatal patients with DRESS syndrome.
Fig. 3.
Comparison of blood lymphocyte counts between steroid-naïve and steroid-used patients with DRESS syndrome.
Fig. 4.
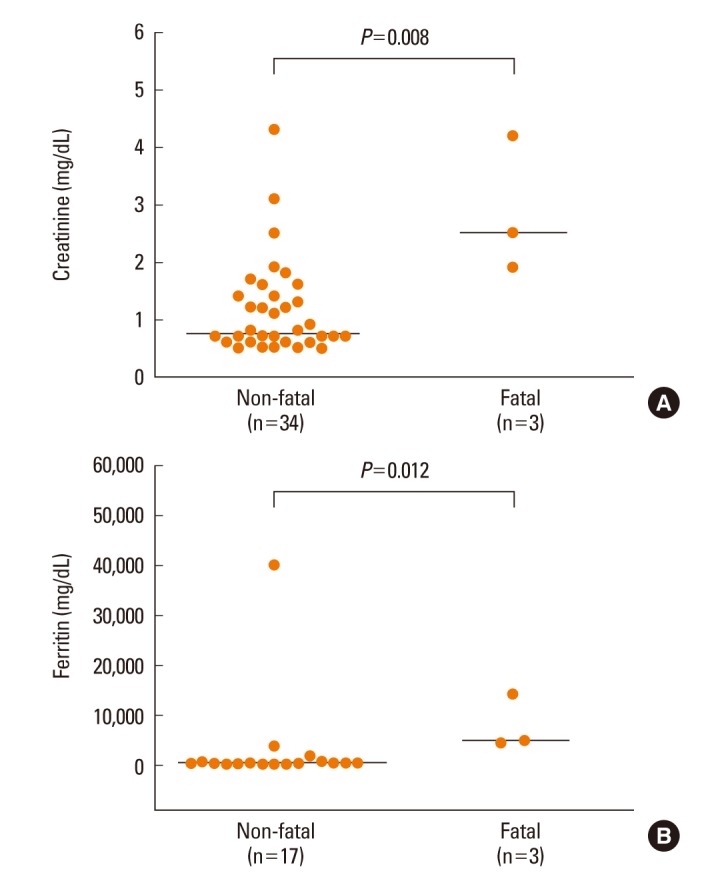
Comparisons of serum creatinine (A) and ferritin (B) levels between non-fatal and fatal patients with DRESS syndrome.
Additionally, further analyses were limited to 28 patients who satisfied RegiSCAR definite criteria. The duration of clinical symptoms was correlated with WBC count (Rs=0.517, P=0.008, n=25) and lymphocyte count (Rs=0.459, P=0.02, n=25) and tended to be related to eosinophil count (Rs=0.314, P=0.12, n=25) in non-fatal cases. Patients who were given systemic steroids showed higher blood lymphocyte counts compared to patients not given steroids (1,650.0 [500.0-5,520.0]/µL, n=20 vs 945.0 [120.0-2,000.0]/µL, n=8; P=0.03). The fatal cases showed higher serum creatinine (2.5 [1.9-4.2] mg/dL, n=3 vs 1.2 [0.5-3.1] mg/dL, n=25; P=0.01) and ferritin (4,744.5 [4,262.0-14,194.4] ng/mL, n=3 vs 354.7 [126.3-40,000.0] ng/mL, n=14; P=0.02) compared to non-fatal cases.
Finally, we analyzed a prognostic factor that contributes to the degree of hepatic injury. In 30 patients with hepatic involvement, peak ALT level during hospitalization was correlated with lymphocyte count (Rs=0.444, P=0.01), but not with other laboratory findings.
DISCUSSION
The diagnosis of DRESS syndrome could be delayed and clinical studies on DRESS syndrome could be interrupted due to different criteria.7 Many investigators have recognized the need for unified diagnostic criteria of DRESS syndrome.7,16 However, it is presently unclear which criteria among Bocquet's criteria,4 the RegiSCAR criteria,6 and Japanese criteria9 are the most suitable to diagnose DRESS syndrome. The present study showed that Bocquet's criteria are simple to use and appropriate for the diagnosis of DRESS syndrome in clinical practice.
The RegiSCAR criteria6 consist of 7 clinical features. Each feature is scored, and total scores are obtained. Patients are classified using a scoring system as no, possible, probable, or definite cases. RegiSCAR definite criteria could be accurate for the diagnosis of DRESS syndrome; however, the complexity of the scoring system makes the criteria difficult to use in clinics. In contrast, Bocquet's criteria4 include just 3 features (which make-the criteria easier for use in practice) and the features have no scoring system. We found that almost all patients who fulfilled RegiSCAR definite criteria satisfied Bocquet's criteria; in addition, most cases that met Bocquet's criteria satisfied RegiSCAR definite criteria. Moderate agreement was observed between Bocquet's criteria and RegiSCAR definite criteria. All patients diagnosed with DRESS syndrome by the Japanese atypical DIHS criteria satisfied Bocquet's criteria. However, a few patients that did not fulfill Bocquet's criteria were diagnosed with DRESS syndrome by RegiSCAR probable criteria. Bocquet's and Regi-SCAR criteria should be complementary for the diagnosis of DRESS syndrome in suspected patients.
A small number of patients satisfied DIHS criteria; therefore, the Japanese DIHS criteria may be too strict to diagnose DRESS syndrome. Japanese DIHS may represent a severe subgroup of DRESS syndrome9 or may be a different disease from DRESS syndrome as defined by RegiSCAR or Bocquet's criteria. The diagnosis of Japanese DIHS requires evidence for the reactivation of HHV-6,9 which is not included in RegiSCAR or Bocquet's criteria. Several herpesviruses (including Epstein-Barr virus, HHV-6, HHV-7, and cytomegalovirus) may play a critical role in the pathogenesis of DIHS.5 However, evidence for HHV-6 reactivation was rare in the present study. It has been stressed that Japanese DIHS is induced by a limited number of specific medications (such as anticonvulsants, allopurinol, and sulfonamides). It is possible that clinical manifestations of DRESS syndrome or DIHS may depend on the type of culprit drug.19 In the present study, we found antibiotics to be the most common causative drugs.
DRESS syndrome is a severe adverse drug reaction that may be fatal in 10% of patients.8 We detected a mortality rate of 8.3%. Additionally, DRESS syndrome is characterized by prolonged clinical symptoms even after the withdrawal of culprit drugs.4 Clinical manifestations persisted for 1 month in our patients with DRESS syndrome. It is necessary to recognize prognostic factors to predict fatality or the duration of clinical symptoms in patients with DRESS syndrome. Several studies have reported possible factors that contribute to fatalities.8,10,20 Chiou et al.8 reported that blood eosinophilia and multiple underlying disorders could be poor prognostic factors related to death from DRESS syndrome. Chen et al.10 reported that thrombocytopenia, a history of chronic renal insufficiency, multi-organ involvement, and pancytopenia are frequently found in fatal cases. Wei et al.20 showed that tachycardia, tachypnea, coagulopathy, gastrointestinal bleeding, systemic inflammatory response syndrome, and leukocytosis are prognostic factors for death. In the present study, we showed that serum levels of creatinine and ferritin at initial presentation could contribute to fatalities in patients who satisfied Bocquet's or RegiSCAR definite criteria. Ferritin is an intracellular protein that migrates to blood as inflammatory reactions occur. It is speculated that increased drug and virus-induced immunological reactions result in the production of more serum ferritin in DRESS syndrome. Severe hepatic involvement may be the most common cause of death in patients with DRESS syndrome. However, hepatic abnormalities were not the cause of death in fatal cases and were not prognostic factor in the present study. Additionally, peak levels of hepatic enzymes measured during hospitalization were unassociated with clinical severity (data not shown). Patients with DRESS syndrome may show great variability in involved target organs and in severity. Life-threatening hepatic involvement may be caused by certain causative drugs.19 Further studies are needed in a larger population.
We analyzed early prognostic factors that contribute to the duration of clinical symptoms. Steroids were used in patients with more severe DRESS syndrome; subsequently, we analyzed factors that influence the requirement for steroid use. Our study showed that lymphocyte and eosinophil counts at initial presentation were significant contributing factors for prolonged clinical symptoms. Additionally, blood lymphocyte counts increased in steroid-using patients compared to steroid-naïve patients, suggesting that higher blood lymphocyte counts may be a poor prognostic factor. The role of blood lymphocyte counts as a prognostic factor may be related to the immunopathogenesis of DRESS syndrome.21 Drug-specific or virus-specific T cells play an important role in the pathogenesis of DRESS syndrome. Anti-viral CD8+ T cells are activated and produce large amounts of tumor necrosis factor-α and interferon-γ in patients with DRESS syndrome, which are increased in patients with the most severe visceral involvement.22 Therefore, blood lymphocyte counts may be associated with DRESS syndrome severity. We also showed that higher blood eosinophil counts could be a poor prognostic factor for prolonged clinical symptoms. Since serum interleukin-5 (IL-5) levels are increased in patients with DRESS syndrome, Th2 cell-derived IL-5 may be involved in drug-induced blood eosinophilia.23 It is possible that blood eosinophilia may be associated with higher blood lymphocyte counts in patients with DRESS syndrome. Further studies are needed to determine whether CD4+ or CD8+ T cells are an important prognostic factor.
Bocquet's criteria may be simple to use and appropriate to diagnose DRESS syndrome in clinical practice. Lymphocyte and eosinophils blood count as well as serum levels of creatinine and ferritin at the onset of DRESS syndrome could be useful prognostic factors.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Wolf R, Orion E, Marcos B, Matz H. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171–181. doi: 10.1016/j.clindermatol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Tas S, Simonart T. Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update. Dermatology. 2003;206:353–356. doi: 10.1159/000069956. [DOI] [PubMed] [Google Scholar]
- 3.Cacoub P, Musette P, Descamps V, Meyer O, Speirs C, Finzi L, Roujeau JC. The DRESS syndrome: a literature review. Am J Med. 2011;124:588–597. doi: 10.1016/j.amjmed.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS) Semin Cutan Med Surg. 1996;15:250–257. doi: 10.1016/s1085-5629(96)80038-1. [DOI] [PubMed] [Google Scholar]
- 5.Shiohara T, Inaoka M, Kano Y. Drug-induced hypersensitivity syndrome (DIHS): a reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol Int. 2006;55:1–8. doi: 10.2332/allergolint.55.1. [DOI] [PubMed] [Google Scholar]
- 6.Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, Roujeau JC. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609–611. doi: 10.1111/j.1365-2133.2006.07704.x. [DOI] [PubMed] [Google Scholar]
- 7.Walsh SA, Creamer D. Drug reaction with eosinophilia and systemic symptoms (DRESS): a clinical update and review of current thinking. Clin Exp Dermatol. 2011;36:6–11. doi: 10.1111/j.1365-2230.2010.03967.x. [DOI] [PubMed] [Google Scholar]
- 8.Chiou CC, Yang LC, Hung SI, Chang YC, Kuo TT, Ho HC, Hu S, Hong HS, Chung WH. Clinicopathological features and prognosis of drug rash with eosinophilia and systemic symptoms: a study of 30 cases in Taiwan. J Eur Acad Dermatol Venereol. 2008;22:1044–1049. doi: 10.1111/j.1468-3083.2008.02585.x. [DOI] [PubMed] [Google Scholar]
- 9.Shiohara T, Iijima M, Ikezawa Z, Hashimoto K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol. 2007;156:1083–1084. doi: 10.1111/j.1365-2133.2007.07807.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen YC, Chiu HC, Chu CY. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol. 2010;146:1373–1379. doi: 10.1001/archdermatol.2010.198. [DOI] [PubMed] [Google Scholar]
- 11.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 12.Venulet J. Role and place of causality assessment. Pharmacoepidemiol Drug Saf. 1992;1:225–234. [Google Scholar]
- 13.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 14.Wi JO, Jin NC, Han ER, Yoon BJ, Park SH, Koh YI. A case of DRESS syndrome accompanied by leukocytoclastic vasculitis. Korean J Asthma Allergy Clin Immunol. 2010;30:320–324. [Google Scholar]
- 15.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 16.Ang CC, Wang YS, Yoosuff EL, Tay YK. Retrospective analysis of drug-induced hypersensitivity syndrome: a study of 27 patients. J Am Acad Dermatol. 2010;63:219–227. doi: 10.1016/j.jaad.2009.08.050. [DOI] [PubMed] [Google Scholar]
- 17.Um SJ, Lee SK, Kim YH, Kim KH, Son CH, Roh MS, Lee MK. Clinical features of drug-induced hypersensitivity syndrome in 38 patients. J Investig Allergol Clin Immunol. 2010;20:556–562. [PubMed] [Google Scholar]
- 18.Jeung YJ, Lee JY, Oh MJ, Choi DC, Lee BJ. Comparison of the causes and clinical features of drug rash with eosinophilia and systemic symptoms and stevens-johnson syndrome. Allergy Asthma Immunol Res. 2010;2:123–126. doi: 10.4168/aair.2010.2.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kano Y, Shiohara T. The variable clinical picture of drug-induced hypersensitivity syndrome/drug rash with eosinophilia and systemic symptoms in relation to the eliciting drug. Immunol Allergy Clin North Am. 2009;29:481–501. doi: 10.1016/j.iac.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Wei CH, Chung-Yee Hui R, Chang CJ, Ho HC, Yang CH, Lin YJ, Chung WH. Identifying prognostic factors for drug rash with eosinophilia and systemic symptoms (DRESS) Eur J Dermatol. 2011;21:930–937. doi: 10.1684/ejd.2011.1550. [DOI] [PubMed] [Google Scholar]
- 21.Camous X, Calbo S, Picard D, Musette P. Drug reaction with eosinophilia and systemic symptoms: an update on pathogenesis. Curr Opin Immunol. 2012;24:730–735. doi: 10.1016/j.coi.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Picard D, Janela B, Descamps V, D'Incan M, Courville P, Jacquot S, Rogez S, Mardivirin L, Moins-Teisserenc H, Toubert A, Benichou J, Joly P, Musette P. Drug reaction with eosinophilia and systemic symptoms (DRESS): a multiorgan antiviral T cell response. Sci Transl Med. 2010;2:46ra62. doi: 10.1126/scitranslmed.3001116. [DOI] [PubMed] [Google Scholar]
- 23.Choquet-Kastylevsky G, Intrator L, Chenal C, Bocquet H, Revuz J, Roujeau JC. Increased levels of interleukin 5 are associated with the generation of eosinophilia in drug-induced hypersensitivity syndrome. Br J Dermatol. 1998;139:1026–1032. doi: 10.1046/j.1365-2133.1998.02559.x. [DOI] [PubMed] [Google Scholar]