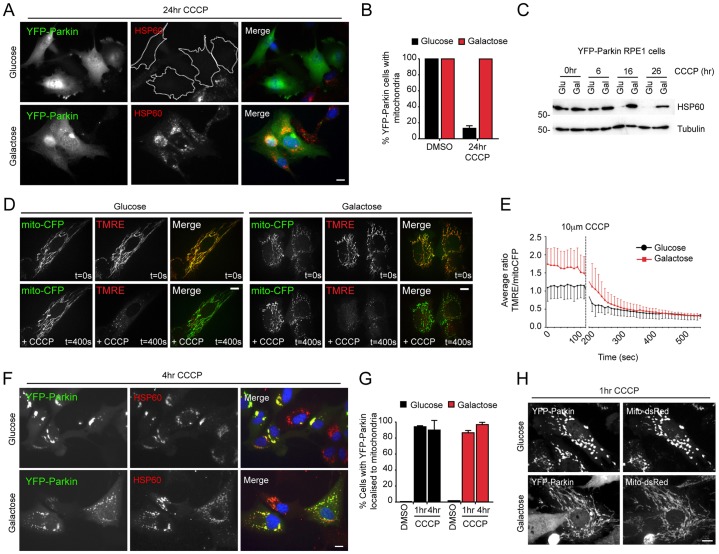
Fig. 2.
Parkin-mediated mitophagy is inhibited in OXPHOS-dependent RPE1 cells. (A,B) Analysis of mitophagy by using wide-field fluorescence microscopy of glucose- and galactose-cultured YFP–Parkin-expressing RPE1 cells that had been treated with 10 µM CCCP (A, example fields; B, quantification; n = 3; means±s.d.). (C) Analysis of mitophagy by immunoblotting for HSP60 in glucose- and galactose-cultured YFP–Parkin-expressing RPE1 cells that had been treated with 10 µM CCCP. (D,E) Δψm dissipation rates in glucose- and galactose-cultured RPE1 cells. (D) Example stills from live-cell movies of glucose- and galactose-cultured RPE1 cells expressing YFP–Parkin and mito–CFP that had been loaded with 50 ng/ml TMRE and treated with 10 µM CCCP (YFP–Parkin not shown). The movies were obtained with a spinning disc confocal microscope. (E) The ratio of TMRE∶mito–CFP was calculated for the mitochondria in each movie, and the means of each timepoint±s.d. are shown (8–9 cells across two experiments). Similar rates of loss of TMRE fluorescence were recorded in cells that had been grown on both energy substrates. (F,G) YFP–Parkin recruitment kinetics were assessed by fluorescence imaging (F, example fields; G, quantification; n = 3; means±s.d.). Note the different distribution of mitochondria that are decorated with YFP–Parkin between conditions (see text). (H) YFP–Parkin-decorated mitochondria remain elongated in OXPHOS-dependent cells at early timepoints after treatment with CCCP. RPE1 cells that stably expressed YFP–Parkin and mito–CFP were imaged by using confocal microscopy. Scale bars: 10 µm.