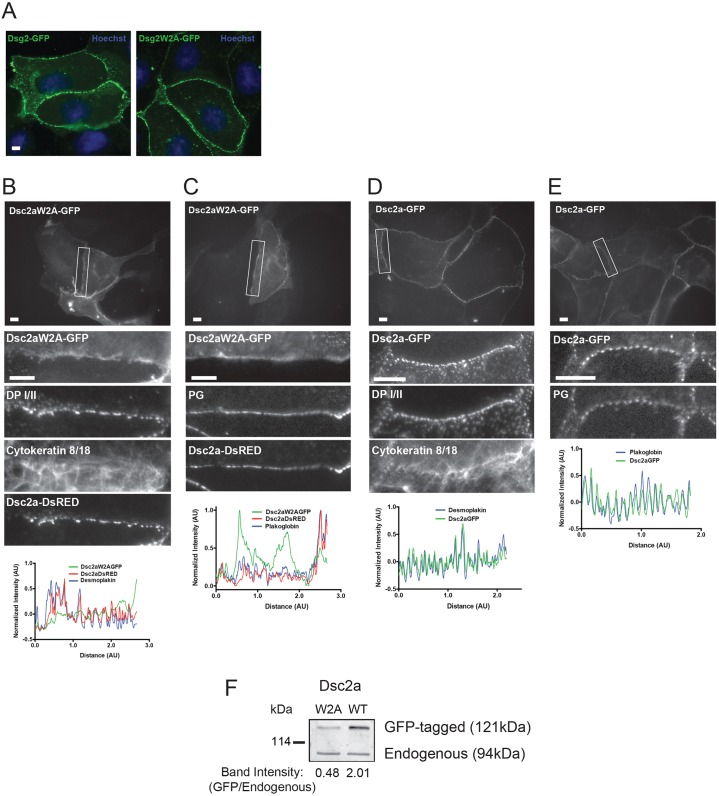
Fig. 4.
The W2A mutation disrupts localization of Dsc2a to punctate complexes. (A) MDCK cells were transiently transfected with wild-type GFP-tagged Dsg2 or GFP-tagged Dsg2W2A and fixed with methanol. Wild-type Dsg2–GFP and mutant Dsg2W2A–GFP localized in characteristic desmosome-like punctate spots at cell–cell contacts. Scale bar: 10 µm. (B–E) MDCK cells were transiently transfected with GFP-tagged Dsc2aW2A or wild-type GFP-tagged Dsc2a, with or without RFP-tagged wild-type Dsc2a, fixed and stained for endogenous desmosomal proteins. Top: a wide-field image with the representative contact outlined in white. Middle: zoomed-in images of the representative cell–cell contacts. Bottom: line scans of normalized fluorescence intensity for Dsc2aW2A–GFP, Dsc2a–GFP, Dsc2a–DsRED, plakoglobin (PG) and DP I/II were plotted for the representative cell–cell contact. Intensity was normalized by subtracting the average intensity over the whole contact and dividing by the difference in maximum and minimum values (pixel range). (B,C) Dsc2aW2A–GFP accumulated in a linear, non-punctate distribution along the plasma membrane and did not co-localize with desmosomal proteins (DP I/II, cytokeratin 8/18, and plakoglobin) or co-transfected wild-type Dsc2a_DsRED, all of which had a characteristic desmosome-like punctate distribution (D,E) Wild-type Dsc2a–GFP localized into punctate spots at cell–cell contacts and colocalized with the desmosomal proteins (DP I/II, cytokeratin 8/18 and plakoglobin). Of note, the focal plane was set using the staining of endogenous proteins as the marker, so as not to create any biases between the transfected GFP-tagged proteins. Scale bar: 5 µm. (F) Expression levels of transiently transfected cells (similar efficiencies with >60% transfected) Dsc2aW2A–GFP were ∼1/2 that of endogenous Dsc2a in MDCK cells.