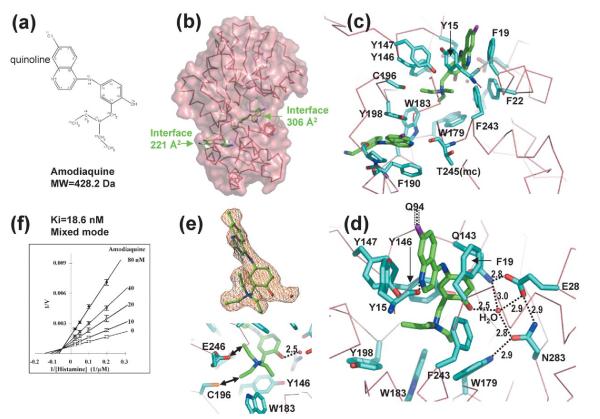
Figure 4.
Interactions of HNMT and amodiaquine. (a) Chemical structure of amodiaquine. (b) Two molecules of amodiaquine bind per HNMT; one binds in the active site pocket and the other in an outer-surface pocket. (c) Detailed plots of HNMT–amodiaquine interactions. The branch structure of amodiaquine is disordered in the outer-surface pocket. (d) A network of hydrogen bonds connects the side-chains of Tyr146, Qln143, Glu28, Asn283, Trp179, and a water molecule. The water molecule interacts with the hydroxyl group of the phenyl ring of amodiaquine. The chlorine atom of the quinoline ring of amodiaquine makes van der Waals contacts with Cγ of Gln94. (e) The two alkylene chains rotate every 120°, and make them look like three branches. The simulated-annealing omit electron density map is contoured at 3.5s above the mean (top panel). Besides the surrounding aromatic rings, one branch interacts (indicated by double-ended arrows) with the side-chain of Cys196 and another interacts with Glu246 (bottom panel). (f) Lineweaver–Burke plot. The estimated Ki is 18.6 nM.