Short Summary
Following intravenous (IV) administration, the pharmacokinetics of spectinomycin in rats was found to be on par with its profile in other mammalian species including humans with respect to its overall excretion and half-life at effective concentrations. This study, however, indicates that a small fraction of the spectinomycin dose is retained in peripheral tissues for a prolonged period of time at low concentrations.
Spectinomycin (SPC) is an aminocyclitol antibiotic with broad spectrum antibacterial activity isolated from bacterium Streptomyces spectabilis that selectively targets bacterial ribosomes and remains important in both clinical and veterinary use (Davies et al. 1965). It is active against gram-negative bacteria and has been used for many years for the treatment of uncomplicated anogenital gonorrhea in humans (Novak et al. 1990) as well as bacterial respiratory and enteric infections in veterinary medicine (Ellis and Livingston 1994). SPC is only available in parenteral formulations due to a lack of appreciable oral bioavailability. More recently, it has also gained interest as natural product starting material for the synthesis of new semisynthetic antibiotics with increased target activity and improved pharmacokinetic properties. For example, trospectinomycin, an analog of spectinomycin, is more active than spectinomycin against numerous bacterial species, including staphylococci (MIC 8-32 μg/mL vs. 32->256 μg/mL for SPC), streptococci (MIC 1.0 μg/mL vs. 16 μg/mL for SPC), Haemophilus influenza (MIC 2.0 μg/mL vs. 16 μg/mL for SPC), Neisseria gonorrhoeae (MIC 2.0 μg/mL vs. 32 μg/mL for SPC), and Chlamydia trachomatis (MIC 3.51 μg/mL vs. 250 μg/mL for SPC) (Zurenko et al. 1988).
The rat is widely used as the first preclinical animal model for evaluating and comparing pharmacokinetic profiles of potential drug candidates (Ward and Smith 2004). So far, however, the pharmacokinetic profile of SPC is only available in chicken, sheep, pigs and cattle as well as humans, but has not been reported for the rat. Thus, the purpose of the present study was to evaluate the pharmacokinetics of spectinomycin in rats after intravenous administration as reference point for further development activities on SPC derivatives.
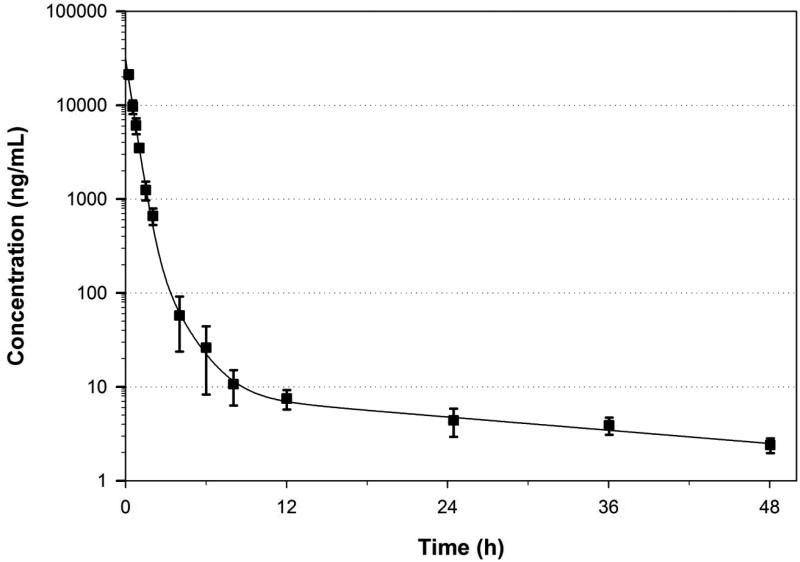
After IV administration of 10 mg/kg SPC plasma concentrations followed a tri-exponential disposition function that was best described by a three-compartment model with a peak plasma concentration of 37.8 μg/mL and a systemic exposure (area-under the curve AUC0-∞) of 15.7 μg h/mL. Between-animal variability in exposure was low with a coefficient of variation of <15% for AUC0-∞. The average measured and model estimated SPC plasma concentration-time profiles are shown in Figure 1, and the corresponding mean pharmacokinetic parameters are listed in Table 1. The relatively rapid initial decline of concentration in the α-phase of the concentration-time profile with a half-life of 0.24 h is representative of rapid distribution to peripheral compartments from the systemic circulation, followed by a slower β-phase characterized by distribution and elimination with a half-life of 0.75 h, and a prolonged γ-phase with a half-life of 19.5 h representing the slow redistribution of SPC from peripheral compartments. The two first phases represent 98% of the AUC0-∞ indicating that the γ-phase does not significantly contribute to the overall drug exposure. The rapid elimination of most of the drug from the body is also reflected by the short mean residence time of 1.1 h. Since plasma concentrations in the γ-phase were 103-104 times lower than the peak concentrations and substantially lower than the minimum inhibitory concentration for the targeted microorganisms, this phase does not contribute to the antibiotic efficacy of SPC. Hence, the β-half-life is the therapeutically relevant half-life for SPC. The average volume of distribution of 0.75 L/kg suggests that distribution is largely limited to the extracellular body water. This observation is supported by the low binding to plasma proteins of only 13.0%.
Figure 1.
Measured and model-predicted plasma concentration-time profile (mean ± standard deviation) of spectinomycin following intravenous administration of 10 mg/kg in rats.
Table 1.
Pharmacokinetic parameters (mean ± standard deviation) of spectinomycin from non-compartmental and compartmental analysis after intravenous administration of 10 mg/kg to rats.
| PARAMETER | NON-COMPARTMENTAL ANALYSIS | THREE-COMPARTMENTAL MODELING# |
|---|---|---|
| C0 (μg/mL) | 44.3 ± 4.1 | 37.8 ± 10.9 |
| AUC0-∞ (μg h/mL) | 16.8 ± 1.8 | 15.7 ± 2.4 |
| Vd (L/kg) | 0.756 ± 0.342 | 0.747 ± 0.218 |
| CL (L/h/kg) | 0.602 ± 0.069 | 0.649 ± 0.103 |
| MRT (h) | 0.757 ± 0.664 | 1.11 ± 0.50 |
| t1/2 α (h) | - | 0.237 ± 0.069 |
| t1/2 β (h) | - | 0.754 ± 0.372 |
| t1/2 γ (h) | - | 19.5 ± 9.0 |
| fe | 0.553 ± 0.144 | |
| CLrenal (L/h/kg) | 0.359 ± 0.086 | |
| Eratio | 1.00 ± 0.29 |
The individual volumes of distribution for the three compartments of the model were 0.278 ±0.059 L/kg, 0.057 ±0.033 L/kg, and 0.412 ± 0.149 L/kg, with distribution clearances among the central and the two peripheral compartments of 0.078 ± 0.023 L/h/kg and 0.013 ± 0.004 L/h/kg, respectively.
The observed disposition behavior with a prolonged retention of a small proportion of the dose in the body is a common disposition pattern for aminoglycosides and many other amines (Mingeot-Leclercq 1990) and is likely related to slow leakage of compound from deep tissue compartments. The underlying mechanism is likely lysosomal trapping and/or complexation with membrane phospholipids (De Broe 1999).
Renal excretion is a major elimination pathway for SPC. Following IV administration, approximately 55% of the drug was excreted into the urine in unchanged form, indicating an approximately equal contribution of renal and non-renal elimination pathways. This is in agreement with previous reports that after IM administration of radiolabelled SPC (5 mg/kg/day) to rats, the majority of the radioactivity was excreted in the urine (54-73%), with the remainder (1-24%) in the feces (Novak et al. 1990; Abu-Basha et al. 2007; Ziv and Sulman 1973; Cuerpo and Livingston 1994). The renal clearance CLrenal was 1.31 mL/min (0.359 L/h/kg) and the non-renal clearance was 1.03 mL/min (0.290 L/h/kg). The resulting renal excretion ratio (Eratio) of SPC was 1.0, suggesting that glomerular filtration without net reabsorption or net secretion is the primary renal elimination process.
With its short β-half-life and rapid elimination from the body, the observed pharmacokinetic profile of SPC in rats is comparable to the data reported for other species. Following single dose intramuscular administration, the overall elimination half-life of SPC was 1.2 h in cattle, 1.0 h in sheep, 1.0 h in pigs, 1.65 h in chicken and 1.85 h in humans, which is comparable to our β half-life of 0.75 h (Abu-Basha et al. 2007). In our study in rats, however, SPC exhibited a tri-exponential distribution patterns with prolonged tissue distribution phase (γ-phase) compared to bi-exponential concentration-time profiles described for the other species. The lack of reports for a prolonged γ-phase in the other species is likely the consequence of a lack of sufficiently sensitive analytical methodology used in some of the previous reports and/or shorter plasma sampling periods.
Experimental
Catheterized male Sprague-Dawley rats (femoral vein for drug administration and jugular vein for blood sample collection) weighing approximately 200-225g were obtained from Harlan Bioscience (Indianapolis, USA). Animals were kept on a 12-hour light/ dark cycle with access to food and water ad libitum. The study protocol was approved by the institutional animal care and use committee of the University of Tennessee Health Science Center. SPC drug solution (10 mg/kg, Sigma Aldrich, USA) was prepared in physiologic saline solution, sterile filtered (0.2 micron syringe filter), and administered intravenously to rats (n=5). Serial blood samples (approx. 250 μL) were collected pre-dose, and at 0.25, 0.5, 0.75, 1.0, 1.5, 2.0, 4.0, 6.0, 8.0, 12.0, 24.0, 36.0 and 48.0 h post-dose. Plasma was separated immediately by centrifugation (10,000g for 5 min at 4°C) and stored at −80°C until analysis. Urine samples were collected at an interval of 0-6, 6-12, 12-24, 24-36 and 36-48 h post-dose and stored at −80°C until analysis.
Plasma and urine samples were analyzed for drug concentrations by an LC-MS/MS assay after protein precipitation with methanol. Chromatographic separations were carried out with a Phenomenex® HILIC, 5 μm C18, 100 × 4.6 mm column (Phenomenex, Torrance, CA) and a gradient of methanol and 10 mM ammonium formate buffer pH ~2.75 at a flow rate of 0.4 mL/min. Detection was performed with an API 3000 triple-quadruple mass spectrometer (Applied Biosystems ABI/MDS-Sciex, Foster City, CA) with electrospray ionization in multiple reaction monitoring mode, using the for SPC characteristic mass transfer of m/z 365.1→333.2. A calibration curve was constructed by spiking SPC into 50 μL of blank rat plasma or urine, using a structurally similar analogue as internal standard. The peak area ratios of analyte to internal standard were linear over a concentration range of 1.5-50,000 ng/mL, with a coefficient of variation of < ±3% for accuracy and < 2% for precision. Plasma protein binding was determined by equilibrium dialysis at 37°C using the RED device (Thermo Scientific, Rockford, USA).
The experimental plasma concentration-time profiles were analyzed by a standard non-compartmental analysis as well as a model-based compartmental analysis using Phoenix-WinNonlin 6.2 (Pharsight Corporation, Mountain View, CA). The fraction (fe) of the test compound excreted in urine was calculated as the cumulative amount of dose excreted unchanged in urine divided by the dose of the test compound administered. Renal clearance (CLrenal) was assessed as product of total clearance (CLtot) and fe , and the renal excretion ratio (Eratio) as the ratio between the product of CLrenal and fu divided by the glomerular filtration rate in rats (1.31 mL/min; Davies and Morris 1993).
Acknowledgement
This work was supported by research grant R01AI090810 by the National Institutes of Health and the American Lebanese Syrian Associated Charities (ALSAC).
References
- Abu-Basha EA, Gehring R, Albwa'neh SJ. Pharmacokinetics and bioavailability of spectinomycin after i.v., i.m., s.c. and oral administration in broiler chickens. J Vet Pharmacol Ther. 2007;30:139–144. doi: 10.1111/j.1365-2885.2007.00825.x. [DOI] [PubMed] [Google Scholar]
- Cuerpo L, Livingston RC. Spectinomycin. Residues of some veterinary drugs in animals and foods monograph, 42nd meeting of the joint FAO/WHO expert committee on food additives, FAO Food Nutr Pap. 1994;41(6):1–86. [PubMed] [Google Scholar]
- Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- Davies J, Anderson P, Davis BD. Inhibition of protein synthesis by spectinomycin. Science. 1965;149(3688):1096–1098. doi: 10.1126/science.149.3688.1096. [DOI] [PubMed] [Google Scholar]
- De Broe ME. Berl T, Bonventre JV, editors. Renal injury due to environmental toxins, drugs, and contrast agents. Atlas of Diseases of the Kidney. 1999;1:11.1–11.6. [Google Scholar]
- Ellis RL, Livingston RC. Spectinomycin. Addendum to the Spectinomycin residue mongraph, 42nd meeting of the Committee, FAO Food and Nutr Pap. 1994;41(6):119–132. [Google Scholar]
- Mingeot-Leclercq MP, Piret J, Brasseur R, Tulkens PM. Effect of acidic phospholipids on the activity of lysosomal phospholipases and on their inhibition by aminoglycoside antibiotics—I: Biochemical analysis. Biochem Pharmacol. 1990;40(3):489–497. doi: 10.1016/0006-2952(90)90547-x. [DOI] [PubMed] [Google Scholar]
- Novak E, Paxton LM, Bye A, Patel R, Zurenko GE, Francom SF. Human safety and pharmacokinetics of a single intramuscular dose of a novel spectinomycin analog, trospectomycin (U-63,366F). Antimicrob Agents Chemother. 1990;34(12):2342–2347. doi: 10.1128/aac.34.12.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward KW, Smith BR. A comprehensive quantitative and qualitative evaluation of extrapolation of intravenous pharmacokinetic parameters from rat, dog, and monkey to humans. I. clearance. Drug Metab Dispos. 2004;32(6):603–611. doi: 10.1124/dmd.32.6.603. [DOI] [PubMed] [Google Scholar]
- Ziv G, Sulman FG. Serum and milk concentrations of spectinomycin and tylosin in cows and ewes. Am J Vet Res. 1973;34:329–333. [PubMed] [Google Scholar]
- Zurenko GE, Yagi BH, Vavra JJ, Wentworth BB. In vitro antibacterial activity of trospectomycin a novel spectinomycin analog (U-63366F). Antimicrob Agents Chemother. 1988;32(2):216–223. doi: 10.1128/aac.32.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]