Abstract
Here, we systematically explore the size and spacing requirements for identifying a letter among other letters. We measure acuity for flanked and unflanked letters, centrally and peripherally, in normals and amblyopes. We find that acuity, overlap masking, and crowding each demand a minimum size or spacing for readable text. Just measuring flanked and unflanked acuity is enough for our proposed model to predict the observer's threshold size and spacing for letters at any eccentricity.
We also find that amblyopia in adults retains the character of the childhood condition that caused it. Amblyopia is a developmental neural deficit that can occur as a result of either strabismus or anisometropia in childhood. Peripheral viewing during childhood due to strabismus results in amblyopia that is crowding limited, like peripheral vision. Optical blur of one eye during childhood due to anisometropia without strabismus results in amblyopia that is acuity limited, like blurred vision. Furthermore, we find that the spacing:acuity ratio of flanked and unflanked acuity can distinguish strabismic amblyopia from purely anisometropic amblyopia in nearly perfect agreement with lack of stereopsis. A scatter diagram of threshold spacing versus acuity, one point per patient, for several diagnostic groups, reveals the diagnostic power of flanked acuity testing. These results and two demonstrations indicate that the sensitivity of visual screening tests can be improved by using flankers that are more tightly spaced and letter like.
Finally, in concert with Strappini, Pelli, Di Pace, and Martelli (submitted), we jointly report a double dissociation between acuity and crowding. Two clinical conditions—anisometropic amblyopia and apperceptive agnosia—each selectively impair either acuity A or the spacing:acuity ratio S/A, not both. Furthermore, when we specifically estimate crowding, we find a double dissociation between acuity and crowding. Models of human object recognition will need to accommodate this newly discovered independence of acuity and crowding.
Keywords: amblyopia, crowding, strabismic, anisometropic, acuity, screening, spacing:acuity ratio, critical spacing, threshold spacing, legibility, overlap masking, letter identification, object recognition
Introduction
Size and spacing limits
Identifying letters is essential to full participation in literate society. Letter identification is a good task for studying object recognition. It is easy to administer and score, yet offers enough distinct responses to evoke categorization rather than mere discrimination. Since Snellen (1866), letter identification has been the main test of vision. The conventional acuity chart is intended to measure the threshold size for identifying an isolated letter. However, “crowding” was discovered in the central vision of amblyopes and the peripheral vision of normals, finding that the measured acuity is affected by the presence of other letters in the vicinity of the target letter (Bouma, 1970; Korte, 1923; Stuart & Burian, 1962). When objects are closer together than the “critical spacing of crowding,” the visual system combines features from them all, producing a jumbled percept. Acuity is a size limit: the smallest readable letter size. Crowding is a spacing limit: What matters is center-to-center spacing, not size. Critical spacing has been measured under a wide range of conditions, but there is no systematic study of how size and spacing together limit legibility of a letter among flankers (neighboring letters). The world is cluttered, so crowding often limits everyday vision more severely than acuity does.
Object recognition, in general, and letter identification, in particular, have been important topics in vision research for a century but are still unexplained. How we recognize an apple or the letter A is still mysterious. Existing proposals are tentative, untested, and not spelled out enough to predict results (flanked acuity) for the very basic task that is the topic of this paper: identifying a letter among others. In this vacuum, based on our results, we propose a model. It is very simple and not computational. It does not explain how we identify. It is a simple rule that specifies the required size and spacing for letter identification. The proposed model allows a new observer's ability to identify letters, at all sizes, spacings, and eccentricities, to be quickly characterized by just two measurements: flanked and unflanked acuity at fixation. We show that the commercially available flanked acuity tests are too loosely spaced to detect overlap masking and suggest that tighter spacing would increase their diagnostic power.
Object recognition can be impaired by nearby objects, flankers. Flankers can prevent object recognition in two ways: crowding and overlap masking. Both are relevant to amblyopia. The two phenomena are very different empirically and theoretically, yet have not always been distinguished in the literature. The spatial extent of crowding is roughly proportional to eccentricity and independent of object size. The spatial extent of overlap masking is roughly independent of eccentricity and proportional to object size.
It has recently been suggested that object recognition is usually limited by spacing, not size (Pelli & Tillman, 2008). Measuring flanked and unflanked acuity with various spacings allows us to expose both the spacing and the size limits. The simple model that describes these data, for both normals and amblyopes, is the first of several conclusions presented here.
In concert with Strappini, Pelli, Di Pace, and Martelli (submitted), we report a double dissociation of acuity and crowding: Different clinical conditions selectively impair one or the other, showing that they are functionally distinct and separately modifiable. Finally, we make recommendations for improved screening for any condition that affects crowding, including amblyopia and apperceptive agnosia.
Modeling amblyopia
Amblyopia is a developmental disorder of vision. It is usually associated with strabismus or anisometropia during early childhood, and these amblyogenic factors are associated with different psychophysical losses (McKee, Levi, & Movshon, 2003). Both strabismic and anisometropic amblyopes have reduced contrast sensitivity and other visual dysfunctions such as reduced Snellen and Vernier acuity, abnormal spatial interaction, and spatial distortions (Ciuffreda, Levi, & Selenow, 1991; McKee et al., 2003). Strabismic and anisometropic amblyopia are different (Hess & Bradley, 1980; Levi & Klein, 1982). The peripheral vision of normals is like the central vision of strabismic amblyopes, and normal peripheral vision has been proposed as a model for strabismic amblyopia (Levi, 1991; Levi & Carkeet, 1993). Purely anisometropic (i.e., non-strabismic) amblyopia, on the other hand, is thought to be a pure acuity loss, since the impairment can be nulled by a task-invariant scaling of stimuli. That is, a single scaling of size and contrast allows an anisometropic amblyope to perform various visual tasks nearly as well as normal individuals do (Hess & Demanins, 1998; Levi, 1991; Levi & Carkeet, 1993). In other words, anisometropic amblyopes see the scaled stimulus nearly as well as normal observers see an unscaled stimulus. Much attention has been given to the ratio of acuity for resolution (e.g., letter or grating acuity) and that for position (e.g., Vernier acuity). That ratio is normal in anisometropic amblyopes and affected in strabismic amblyopes. This has been attributed to “spatial uncertainty” (Levi & Carkeet, 1993).
Amblyopia impairs both detection and identification of a target in clutter (Bonneh, Sagi, & Polat, 2004; Ellemberg, Hess, & Arsenault, 2002; Hess & Jacobs, 1979; Levi & Carney, 2011; Levi, Hariharan, & Klein, 2002a; Levi & Klein, 1985; Polat, Bonneh, Ma-Naim, Belkin, & Sagi, 2005). Bonneh et al. (2004) compared flanked and unflanked letter acuities in a large cohort of amblyopes, finding that flanked acuity is highly correlated with unflanked acuity in purely anisometropic amblyopia but not in strabismic amblyopia. Flanker effects in amblyopic central vision are not yet well understood and are explored systematically here. Based on the above discussion of existing models for strabismic and anisometropic amblyopia, here we ask how well eccentricity or blur in a normal eye mimics the brain effects of strabismic and anisometropic amblyopia.
Here we propose a simple model for letter recognition in central, peripheral, and amblyopic vision and show that it accurately describes letter recognition in strabismic amblyopia by increased eccentricity and in purely anisometropic amblyopia by increased blur. This has practical implications for diagnosis and screening.
Screening for crowding and amblyopia
The childhood development of crowding and that of reading may be linked (Atkinson, 1991; Atkinson, Anker, Evans, Hall, & Pimm-Smith, 1988; Atkinson, Pimm-Smith, Evans, Harding, & Braddick, 1986; Kwon, Legge, & Dubbels, 2007; Pelli & Tillman, 2008) making it potentially useful to have a convenient clinical test for crowding to diagnose this cause for slow reading.
Amblyopia is a nontrivial handicap affecting 3% of the population. Acuity, and sometimes reading speed, are impaired in the affected eye. Stereopsis may be absent. It is treatable if detected early—the earlier the better—and the cost-benefit of early testing and treatment is very favorable (Atkinson, Braddick, Nardini, & Anker, 2007; Dutton & Cleary, 2003; Joish, Malone, & Miller, 2003; but see Snowdon & Stewart-Brown, 1997). The main diagnostic criterion for amblyopia is poor acuity, so it is not surprising that most screening tests for amblyopia are acuity tests (Simmers, Gray, & Spowart, 1997; Simons, 1996, 2005; one exception is the random dot stereogram test, Simons & Moss, 1981).
It is well established that acuity for “surrounded” optotypes (i.e., flanked acuity) is much more sensitive to strabismic amblyopia than is acuity for “isolated” optotypes (unflanked acuity, Simons, 1983; Thomas-Decortis, 1959). Fifty years ago, in Europe, they were called “morphoscopic” (flanked) and “angular” (unflanked) acuity. In charts, the other letters act as flankers, but a single target letter can be surrounded by flankers, as in Figure 1.
Figure 1.

Unflanked and flanked letter identification tasks. The observer is asked to fixate the center of the fixation mark and to identify the target letter, once it appears. The target letter is subsequently presented, briefly, either alone (unflanked) or surrounded (flanked) by four random letters of the same size. The center-to-center letter spacing in degrees between the target and the flankers scales with letter size and is s times the letter size, where s is usually 1.1. In each case, we use an adaptive procedure (QUEST) to determine the threshold size (covaried with spacing) for 50% correct identification. The letter spacing factor s is 1.1× for all data reported in all tables and figures, except where we indicate otherwise (in Tables 1 and 3 and Figures 2 and 3).
As discussed at the end, our findings suggest that the sensitivity of current screening tests for strabismic amblyopia can be increased by making the flankers closer and more similar to the target.
Our study
Here we present systematic measurements of the size and spacing requirements for legibility of a flanked letter, which suggest a simple model for how legibility is limited by acuity, crowding, and overlap masking. We also show that eccentric viewing by a normal eye is a good model for the central vision of strabismic amblyopia and that blur is a good model for anisometropic amblyopia. The double dissociation of acuity and crowding (by purely anisometropic amblyopia and apperceptive agnosia) shows that crowding and acuity are “functionally distinct and separately modifiable” aspects of letter identification. (The quote comes from Sternberg's 2003 treatise on process decomposition by double dissociation.) Finally, we consider implications for screening.
Methods
Stimuli were generated by an Apple G4 PowerBook using MATLAB with the Psychophysics Toolbox extensions and presented on a gamma-corrected Sony G400 monitor with the (green) background luminance set to 30 cd/m2, the middle of the monitor's range (Brainard, 1997; Pelli, 1997).
Observers
Twenty-one observers participated in our study. Eighteen were amblyopic: six were just strabismic, six were just anisometropic, and six were both strabismic and anisometropic. Three observers had normal vision. Amblyopia is diagnosed when a complete eye examination reveals that the best corrected visual acuity is either poorer than 20/30 or is at least two lines (0.2 log units larger) worse than that of the contralateral eye, in the absence of any obvious structural anomalies or pathologic signs. We classified an amblyope as purely anisometropic if associated with amblyogenic anisometropia but not strabismus and as strabismic if associated with an early onset, constant, and unilateral deviation at both near and far, whether or not they had anisometropia. In the text that follows we will often describe observers as either strabismic or purely anisometropic; however, in the figures we will separately color-code amblyopes with pure anisometropia (green), pure strabismus (red), and strabismus with anisometropia (blue), following the color code used by McKee et al. (2003). The detailed characteristics of each of the normal and amblyopic observers are listed in Table 1. All observers except VC and SF have central or near central (<0.5°) steady fixation, as determined via visuoscopy. Strabismic-and-anisometropic observer VC has large eccentric fixation (about 8°) in her amblyopic (right) eye. Strabismic amblyopic observer SF has unsteady eccentric fixation in his amblyopic (left) eye. In order to examine SF's fixation pattern more carefully, we recorded a highly magnified view of his retina as he attempted to fixate a 6 × 6 min cross presented at the center of a 2.5° × 2.5° field for 20 s, using an Adaptive Optics Scanning Laser Ophthalmoscope (Roorda, Romero-Borja, Donnelly, & Queener, 2002). This method allows the target image to be superimposed synchronously on the retinal image and makes it possible to accurately locate the target relative to the fovea. This recording shows that SF initially fixated within 0.5° nasal to the fovea (right of the fixation). Fixation was maintained near the fovea for about 3 s and then drifted up to several degrees nasal-ward (see Discussion). The amblyopic eye of each amblyopic observer and the preferred eye of each normal observer were tested monocularly. All normal observers had or were given substantial experience (hundreds or thousands of trials) in experiments that required peripheral viewing. The experimenter monitored every observer's eye position to ensure that fixation was maintained, discarding the few trials in which it was not, which comprise less than 5% of the total number of trials.
Table 1.
Observer characteristics. The rightmost seven columns (threshold spacing, acuity, spacing:acuity ratio, equivalent blur, equivalent eccentricity, φA, and φcrowding) are plotted in Results. The “Line letter VA” chart has spacing factor 2×. Threshold spacing S was measured with a spacing factor of 1.1×. The add-on eccentricities φcrowding and φA are computed by Equations 15 and 16. Equation 15 shows that crowding is linearly related to eccentricity φ and proportional to “padded” eccentricity φ + φcrowding. So padded eccentricity is a good scale against which to examine crowding effects. The equivalent eccentricity for crowding is given by Equation 2, φeq = φcrowding – 0.45°. Stereopsis was measured using the Randot "Random Dot Geometric Form" test (Stereo Optical Co.).
| Observer |
Age (yrs) |
Strabismus (at 6 m) |
Eye |
Refractive error (diopters, D) |
Line letter VA (single letter VA) |
Stereo* |
Threshold spacing S (deg) |
Acuity size A (deg) |
Spacing:acuity ratio S/A |
Eq. blur (D) |
Eq.ecc. φeq (deg) |
φA (deg) |
φcrowding (deg) |
| Normal | |||||||||||||
| AF | 22 | None | R | +0.25/-0.50 × 180 | 20/12.5 | 20” | — | — | — | — | — | — | — |
| L | +0.25/-0.25 × 25 | 20/12.5 | 0.123 | 0.083 | 1.48 | 0 | 0 | 2.86 | 0.45 | ||||
| EJ | 21 | None | R | −2.50/-0.50 × 83 | 20/12.5 | 25” | — | — | — | — | — | — | — |
| L | −3.00 | 20/12.5 | 0.190 | 0.143 | 1.33 | 0.33 | 0 | 4.93 | 0.45 | ||||
| SS | 28 | None | R | −0.25 | 20/12.5 | 20” | 0.138 | 0.103 | 1.34 | 0.13 | 0 | 3.55 | 0.45 |
| L | pl/-0.25 × 119 | 20/12.5 | — | — | — | — | — | — | — | ||||
| Purely strabismic (not anisometropic) | |||||||||||||
| JS | 22 | L EsoT 6-8Δ & HyperT 4-6Δ | R | +1.25 | 20/16 | fail | — | — | — | — | — | — | — |
| L | +1.00 | 20/40 (20/32+1) | 0.257 | 0.102 | 2.52 | 0.15 | 0.41 | 3.52 | 0.86 | ||||
| SF | 19 | L ExoT 6Δ | R | −1.50/-0.25×90 | 20/12.5+1 | fail | — | — | — | — | — | — | — |
| L | pl/-1.00×30 | 20/125+1 (20/80+2) | 0.993 | 0.523 | 1.90 | 1.58 | 2.86 | 18.03 | 3.31 | ||||
| GW | 58 | R EsoT 4-6Δ | R | pl | 20/63−2 (20/24+2) | fail | 4.398 | 0.353 | 12.46 | 1.05 | 14.21 | 12.17 | 14.66 |
| L | +0.50/-0.75×180 | 20/16−1 | — | — | — | — | — | — | — | ||||
| JZ | 19 | L EsoT 4Δ | R | pl/-0.50×95 | 20/16+2 | fail | — | — | — | — | — | — | — |
| L | −0.25/-0.50×50 | 20/63+2 (20/32−1) | 0.440 | 0.173 | 2.54 | 0.45 | 1.02 | 5.97 | 1.47 | ||||
| BN | 22 | L EsoT 3-4Δ | R | +5.50/-2.25×5 | 20/16+2 | fail | — | — | — | — | — | — | — |
| L | +5.50/-1.50×175 | 20/50−2 (20/25−2) | 0.638 | 0.210 | 3.04 | 0.58 | 1.68 | 7.24 | 2.13 | ||||
| CL | 19 | R EsoT 4Δ | R | −0.75 | 20/50+2 (20/32−2) | fail | 0.538 | 0.255 | 2.11 | 0.73 | 1.34 | 8.79 | 1.79 |
| L | −0.25/-0.50×55 | 20/16−2 | — | — | — | — | — | — | — | ||||
| Strabismic & anisometropic (These data are blue in Figures 2–6 & 11, and red in Figures 8–10 & 12.) | |||||||||||||
| SM | 55 | Alt. ExoT 18Δ | R | +2.75/-1.25 × 135 | 20/40 (20/25+1) | 320” | 0.455 | 0.208 | 2.19 | 0.57 | 1.07 | 7.17 | 1.52 |
| L | −2.00 | 20/16−2 | — | — | — | — | — | — | — | ||||
| JD | 19 | L EsoT 3Δ | R | +2.50 | 20/16 | fail | — | — | — | — | — | — | — |
| L | +5.00 | 20/125 (20/125+2) | 0.765 | 0.413 | 1.85 | 1.24 | 2.1 | 14.24 | 2.55 | ||||
| AW | 22 | R EsoT 4-6Δ & HypoT 4Δ | R | +2.75/-1.0 × 160 | 20/80−1 (20/50−1) | fail | 0.638 | 0.258 | 2.47 | 0.74 | 1.68 | 8.90 | 2.13 |
| L | −1.00/-0.50 × 180 | 20/16−1 | — | — | — | — | — | — | — | ||||
| GJ | 23 | R EsoT 4-5Δ | R | +3.50/-1.00 × 97 | 20/63+1 (20/40−1) | fail | 0.805 | 0.200 | 4.03 | 0.55 | 2.23 | 6.90 | 2.68 |
| L | Pl | 20/16−1 | — | — | — | — | — | — | — | ||||
| AP | 24 | L EsoT 4-5Δ & HyperT 2-3Δ | R | −1.25/-0.50 × 175 | 20/16−2 | fail | — | — | — | — | — | — | — |
| L | −0.50/-0.25 × 60 | 20/50+1 (20/40+2) | 0.580 | 0.225 | 2.58 | 0.63 | 1.48 | 7.76 | 1.93 | ||||
| VC | 23 | R ExoT 5-6Δ | R | +4.25/-1.50×10 | 20/200−4 (20/125−1) | fail | 17.740 | 0.958 | 18.52 | 2.92 | 58.68 | 33.03 | 59.13 |
| L | pl/-0.50×170 | 20/12.5−1 | — | — | — | — | — | — | — | ||||
| Purely anisometropic (non-strabismic) | |||||||||||||
| SC | 27 | None | R | +0.50 | 20/16+2 | fail | — | — | — | — | — | — | — |
| L | +3.25/-0.75 × 60 | 20/50+2 (20/40−2) | 0.332 | 0.220 | 1.51 | 0.61 | 0 | 7.59 | 0.45 | ||||
| CJ | 22 | None | R | −15.00/-1.25 × 150 | 20/125−4 (20/125+1) | 200” | 0.936 | 0.512 | 1.83 | 1.55 | 0 | 17.66 | 0.45 |
| L | −6.00 | 20/16−2 | — | — | — | — | — | — | — | ||||
| AM | 48 | None | R | +0.75/-0.70 × 95 | 20/12.5 | 200” | — | — | — | — | — | — | — |
| L | +2.00 | 20/32−2 (20/25−2) | 0.240 | 0.200 | 1.20 | 0.55 | 0 | 6.90 | 0.45 | ||||
| RA | 15 | None | R | −2.00/-1.75 × 155 | 20/32+1 (20/32+1) | 70” | 0.423 | 0.253 | 1.67 | 0.72 | 0 | 8.72 | 0.45 |
| L | +0.25/-0.25 × 60 | 20/12.5 | — | — | — | — | — | — | — | ||||
| SWP | 24 | None | R | −0.25 | 20/16+2 | 30” | — | — | — | — | — | — | — |
| L | +1.75/-0.25 × 45 | 20/32+2 (20/32+2) | 0.383 | 0.250 | 1.53 | 0.72 | 0 | 8.62 | 0.45 | ||||
| SW | 42 | None | R | +2.00/-0.50 × 90 | 20/12.5−2 | 200” | — | — | — | — | — | — | — |
| L | +4.25/-1.25 × 120 | 20/80−2 (20/63−1) | 0.675 | 0.493 | 1.37 | 1.49 | 0 | 17.00 | 0.45 | ||||
Unflanked and flanked letter identification
For each amblyopic observer, we tested the amblyopic eye and measured the threshold size for letter identification, with and without flankers, with central viewing. For each normal observer, the same measurements were made at five different eccentricities.
Acuity A is the threshold size (in degrees) without any flanker (not its reciprocal, one over size). Acuity A is the size limit. Flanked acuity, A', is the threshold letter size in the presence of flankers. Acuity and flanked acuity are both measured by reducing the stimulus size to reach a criterion level of performance. In our paradigm, the spacing of a flanked letter is a fixed multiple s of the letter size. Thus, at the flanked acuity threshold, one can arbitrarily report either the letter size A' or the letter spacing S, where S = sA' is the threshold spacing (in degrees), center to center, between target and flanker, where s is the spacing factor (multiple of the letter size). In Results we report the (unflanked) acuity A and the threshold spacing S. Our measurement of flanked and unflanked acuity to test for crowding is computerized, which allows us to vary size and spacing systematically and control duration but is otherwise similar to some printed tests, such as Tommila's (1972) flanked and unflanked tumbling E charts, the Cambridge Crowding Cards (Atkinson et al., 1988; Atkinson et al., 1986), and the Glasgow Acuity Cards (McGraw & Winn, 1993).
The fixation mark consisted of four black diagonal lines (0.1° thick) forming an X (2° wide and 2° high) with a 1° diameter gap in the center. The same fixation mark was used for both central and peripheral viewing. Observers were instructed to fixate the invisible intersection point of the lines. In the unflanked letter identification task, the target, a single letter, was presented for 200 ms, either centered at fixation or in the lower visual field at one of these eccentricities: 1.25°, 2.5°, 5°, 10°. In the flanked letter identification task, the target, the letter to be identified, was flanked by four letters (above, right, below, and left). All five letters were presented in the same manner as the unflanked letter (Figure 1). The target (and each flanker, if present) was randomly selected (with replacement) from nine letters of the Sloan alphabet DHKNORSVZ, displayed at 60% contrast as a bright green letter on a dimmer green background. (The Sloan font is available, free for research purposes, at http://psych.nyu.edu/pelli/software.html.) We omit the letter C from the Sloan alphabet because C and O are much less discriminable than any other pair of letters in that alphabet (Elliott, Whitaker, & Bonette, 1990). Letter contrast is defined as the ratio of luminance increment to background. Each presentation was initiated by clicking the mouse and followed by a response screen showing the nine possible choices for the target. The observer identified the target by using a mouse-controlled cursor to point and click on the chosen answer. Correct identification was rewarded by a beep.
The Sloan font is uppercase only. Every letter in each trial has the same height and width, which we take as the letter size. For flanked letters, the center-to-center spacing between any flanker and the central letter is the letter size multiplied by s. The letter spacing factor s is 1.1 unless indicated otherwise. (The unflanked case may be designated s = ∞.) In our experiments, we adjusted the letter size using QUEST to measure the threshold size for unflanked and flanked letter identification. Letter spacing covaried with size to maintain the letter spacing factor s. Our threshold criterion is 50% correct. Each run of 40 trials of the same condition yields one threshold estimate for that condition. Thresholds presented here are the geometric means of estimates from at least four runs.
Using a fixed spacing factor s, our threshold measurement procedure covaries the spacing and size when measuring threshold for flanked letter identification. This is an efficient way to estimate critical spacing (Levi, Song, & Pelli, 2007). Critical spacing Scritical is the minimum center-to-center spacing between the target and flankers that eliminates (or nearly eliminates, depending on the threshold criterion) the effect of the flankers (Bouma, 1970). As we will see in Results, in peripheral vision, threshold spacing equals critical spacing over a broad range of letter spacing (tight to loose, 1.1 ≤ s < 4), but threshold spacing can exceed critical spacing when letter spacing is extremely loose (s > 4). In that case (s > 4) the flankers have no effect and the threshold spacing is limited only by acuity, S = sA. In contrast, in the normal fovea and in purely anisometropic amblyopia, threshold spacing equals critical spacing only when the letter spacing is tight. For this reason, in order to estimate the two limits, in most of the experiments described below we measure threshold size for both s = 1.1 and s = ∞, i.e., both A' and A. As is evident in Figure 2, in order to ensure that performance is limited by spacing (rather than target size) in the normal fovea, the spacing factor s must be small (e.g., 1.1).
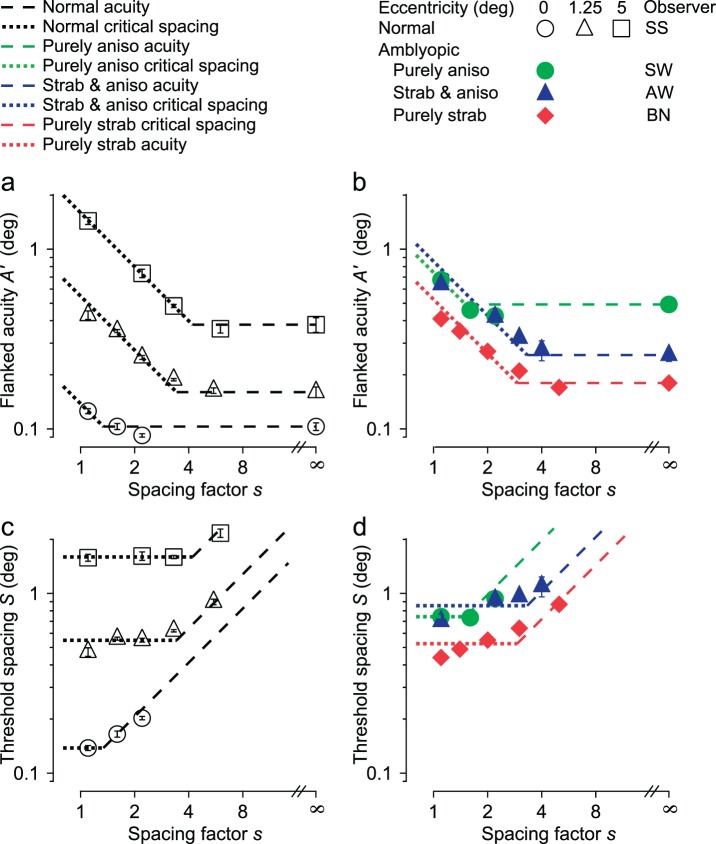
Figure 2.
Flanked letter acuity (a & b) or threshold spacing (c & d) versus letter spacing factor (multiple of the letter size) for normal (a & c) and amblyopic (b & d) observers. Thresholds for the normal observer are measured at 0° (circle), 1.25° (triangle), and 5° (square) eccentricities; thresholds for the purely anisometropic amblyope (green) and the strabismic amblyopes (red, blue) are measured at fixation. The error bars on each data point indicate plus-or-minus one standard error. The same data and models are plotted twice: as flanked acuity A' in the upper graphs (a & b) and as threshold spacing S in the lower graphs (c & d). Threshold spacing S = sA' is the product of the letter spacing factor s and the flanked acuity A'. For each observer, the horizontal line (dashed) in the upper graphs (a & b) is the (unflanked) acuity A (rightmost point), and the horizontal line (dotted) in the lower graphs (c & d) is the critical spacing Scritical, estimated as the geometric mean of the points that lie above the (extended) dashed line. The data and lines are converted back and forth between upper and lower graphs by the relation log S = log s + log A'.
Spacing
Flanked acuities with larger flanker-target spacings were measured at fixation, at 1.25° and 5° eccentricities of one normal observer (SS), and at the fixation of both one purely anisometropic-amblyopic observer (SW) and one strabismic-and-anisometropic amblyopic observer (AW). The center-to-center spacing factors s (multiples of the letter size) between flanker and target that we used are: 1.1, 1.6, and 2.2 at 0° (fixation), 1.1, 1.6, 2.2, 3.3, and 5.5 at 1.25°, and 1.1, 2.2, 3.3, and 6 at 5° for SS; 1.1, 1.6, and 2.2 for SW; and 1.1, 2.2, 3, and 4 for AW. The procedure is as described above for flanked letter identification. For every experiment, we plot threshold spacing S. For this particular experiment, we also plot the flanked acuity A'. Threshold spacing S = sA' is the product of the spacing factor s (multiple of the letter size) and the flanked acuity A'. (The threshold measurement procedure adjusts the size of the whole stimulus to achieve criterion performance. It is entirely arbitrary whether one chooses to take the letter size or spacing as the outcome measure.)
Timing
The temporal sequence of presentations in a trial is: fixation mark (until 400 ms after the observer's “I'm ready” click), stimulus (200 ms), and response screen (until the observer's response click). The stimulus consists of the target, with or without flankers.
These experiments were conducted over three years and some aspects evolved over that time. Initially we used the same methods as in our previous paper (Levi, Song, & Pelli, 2007). At that time, the fixation mark stayed on continuously through the stimulus presentation until it was overwritten by the response screen. Later, when measuring very poor acuities (e.g., with high optical blur), we found that a very large target would overwrite parts of the (fixed size) fixation mark, so we changed our procedure to remove the fixation mark before displaying the target. We have compared thresholds measured in the two ways (fixation mark present or absent during the target presentation) for key conditions, both with and without flankers, and find no difference, so we do not distinguish them in the results reported here.
There were no temporal gaps in the sequence, so it seemed possible, though unlikely, that there might be forward masking from the fixation mark or backward masking from the response screen. That concern can be put to rest: Introducing a 300-ms blank screen between the fixation mark and the stimulus and another 300-ms blank screen between the stimulus and the response screen had no effect on thresholds for the key conditions we tested.
Optical blur
One normal observer, SS, repeated the unflanked and flanked (at the spacing of 1.1 times the letter size) letter identification tasks at 0°, 1.25°, and 5° eccentricities while wearing blurring lenses (net +0.25, +0.50, +0.75, +1.00, +1.50, +3.00, +6.00, or +8.00 D for foveal viewing at the distance of 5, 1, or 0.56 m; net +0.50, +0.75, or +1.00 D for 1.25° eccentric viewing at the distance of 2.5 m; and net +0.50 or +1.00 D for 5° eccentric viewing at the distance of 1 m) in order to investigate the effect of optical blur. Two strabismic-and-anisometropic amblyopic observers, AP and GJ, and one purely anisometropic-amblyopic observer, SW, also repeated the same tasks at fixation while wearing a blurring lens that has the corrective refractive power plus an extra refractive power (net +1.00 or +1.25 D for AP, net +0.75 or +1.25 D for GJ, and net +0.50 or +1.25 for SW) at the viewing distance of 2.5 m. The procedure is the same as described above for the unflanked and flanked letter identification.
Pinhole
We also measured acuities with a small artificial pupil (1.5-mm diameter) both for normal observer SS with a 0, +1, or +1.5 D lens and for a purely anisometropic amblyope SW with a corrective lens.
Pupil size
The optical defocus produced by our blurring lenses increases the size of the eye's point spread function in the retinal image (Campbell & Gubisch, 1966). Optically, that point spread function determines how blurry the retinal image is. The size of the point spread function resulting from a given amount of defocus (in diopters) depends on the pupil size. Depth of field (i.e., tolerance to defocus) is greater at smaller apertures because more defocus (D) is required to produce the same blur in the retinal image. Pupil size affects the optical blur and the retinal illuminance, both of which affect the target's visibility.
Of course, the optical aperture is different when we compare thresholds with and without the 1.5-mm pinhole. The pinhole improved the optical MTF (Modulation Transfer Function) but reduced retinal illuminance by roughly fivefold. To deconfound the two effects of the pinhole, we separately used a neutral density filter (density 0.7, 20% transmission) combined with an artificial pupil (3.5 mm) to assess the isolated effect of reducing retinal illuminance.
Most of our conclusions are based on comparisons of visual functions under flanked and unflanked conditions, and a change in pupil size between these two conditions would make it hard to compare them, but we have avoided that problem. Thus our main conclusions rest on comparisons of measurements within individuals tested at the same luminance doing flanked and unflanked identification in alternate runs. All experiments were performed with the same dim overhead lighting. We are confident that each observer's pupil size (3–4 mm) is not significantly different between flanked and unflanked trials (see Discussion).
Results
The subsection numbers here correspond to the numbering of the final Summary and conclusions section.
1. Size and spacing
We begin with a systematic exploration of the size and spacing requirements for identifying a flanked letter by a normal observer at three eccentricities (Figure 2a, c) and three amblyopes at fixation (Figure 2b, d). The horizontal scale is the letter spacing factor s, the center-to-center letter spacing expressed as a multiple of letter size. In the upper graphs (Figure 2a, b), the vertical scale is flanked acuity A'. In the lower graphs (Figure 2c, d), it is threshold spacing S. Upper and lower graphs are different views of the same data, S = sA'. In the log-log coordinates of Figure 2, each dashed line represents a size limit (i.e., acuity A), and each dotted line represents a spacing limit due to crowding or masking. The lines have been trimmed in the graphs so that only the higher (more severe) limit is shown. The data points are all close to the higher limit, showing that the two limits, size and spacing, together account for all the data,
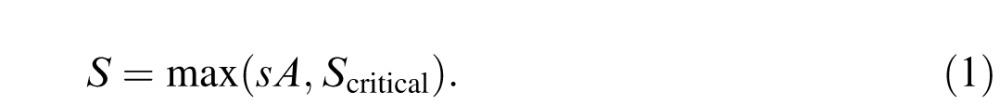 |
This model has two degrees of freedom (acuity A and critical spacing Scritical) for each observer at each eccentricity. The spacing factor s of our test ranged from 1.1 to about 4 and ∞.
Normal vision
In the normal fovea (Figure 2a, b, open circles), vision is mostly limited by acuity and is limited by spacing (dotted line) only at the smallest spacing factor (s = 1.1), whereas peripheral vision is limited by spacing at all measured spacings, which go up to s ≈ 4.
Amblyopes
The amblyopic results (Figure 2b, d) are well fit by the same model (with adjusted parameters) and anticipate some of our other findings for amblyopia. Comparing left and right, we find that the two strabismic amblyopes' results at fixation (filled triangles and diamonds in Figure 2b, d) are much like those of the normal observer viewing eccentrically (1.25° in this case, open triangles in Figure 2a, c). The purely anisometropic amblyope's results (filled circles in Figure 2b, d) are like those of the normal at zero eccentricity (open circles in Figure 2a, c) but shifted up (in these log coordinates) to higher (worse) flanked acuity and threshold spacing.
Acuity and crowding are well-known limits, but this is the first investigation of how they combine to limit letter identification. There seems to be no interaction, since the data are well fit by our model: a letter is legible if and only if it respects both limits.
2. Threshold spacing
Threshold spacing extends over only a few minutes of arc in the normal fovea but extends over degrees in peripheral and amblyopic vision (e.g., Flom, Weymouth, & Kahnemann, 1963; Hariharan, Levi, & Klein, 2005; Hess & Jacobs, 1979; Levi, Hariharan, & Klein, 2002a, 2002b; Levi & Klein, 1985). Threshold spacing can be limited by overlap masking, which is proportional to acuity, or by crowding, which is not. Unlike crowding, overlap masking is independent of eccentricity and its center-to-center extent scales with the size of the signal (Levi & Carney, 2011; Levi, Klein, & Hariharan, 2002; Pelli, Palomares, & Majaj, 2004). Many past accounts did not distinguish overlap masking and crowding.
For example, Flom et al. (1963) estimated the extent of “crowding” in normal and amblyopic observers and reported that the extent of “crowding” was proportional to acuity. This predicts that threshold spacing will be proportional to acuity (see also Levi, Waugh, & Beard, 1994). This prediction is compatible with our overlap masking results (in normal fovea with blur and anisometropic amblyopia) but is incompatible with our crowding results (in normal periphery and strabismic amblyopia).
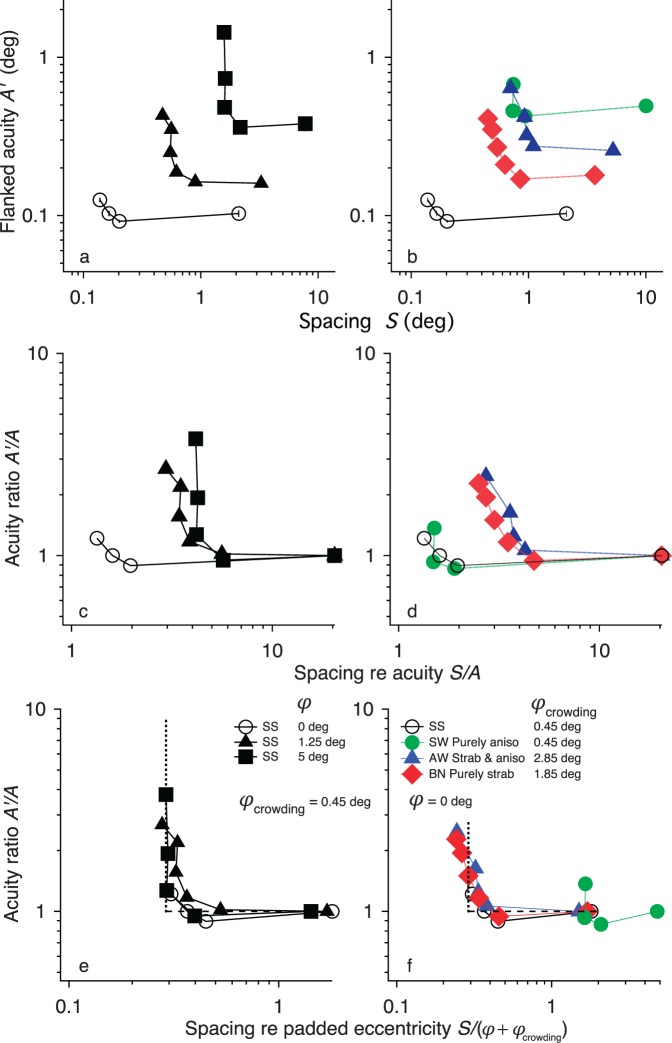
Let us examine how threshold spacing grows with eccentricity. Figure 3 replots the data of Figure 2 in several ways for our normal and amblyopic observers. Figure 3 plots results for normal observers in the left column and amblyopes in the right column. The top row plots flanked acuity A' versus spacing S. This shows that flanked acuity is degraded out to much greater spacing in both peripheral and amblyopic vision than in the fovea. In the second row, we express the effect of flankers as the ratio A'/A, and we assess the Flom et al. (1963) proportionality hypothesis by normalizing spacing by acuity, S/A. The graphs show that this scaling does work for normal central vision and the purely anisometropic amblyope (green in Figure 3d) but does not work for peripheral (black, Figure 3c) or strabismic amblyopic vision (blue or red, Figure 3d): Even after normalizing by acuity, critical spacing is larger in peripheral vision (Figure 3c) and strabismic amblyopia (Figure 3d). That is, threshold spacing in peripheral vision or strabismic amblyopia is much worse than predicted by proportionality to acuity. This parallels the old finding for peripheral and strabismic amblyopic vision that Vernier acuity worsens more than in proportion to acuity (Levi & Carkeet, 1993).
Figure 3.
Scaling. Replotted from Figure 2. The parameters are acuity A, flanked acuity A', spacing S, and eccentricity φ. Left: normal vision; Right: amblyopia. Top: A' versus spacing S; Middle: normalized by acuity, plotting the acuity ratio A'/A versus spacing:acuity ratio S/A; Bottom: the acuity ratio A'/A versus spacing normalized by padded eccentricity S / (φ + φcrowding). For normals (left) φcrowding = 0.45° and several eccentricities φ are tested. For the amblyopes (right) the eccentricity is zero φ = 0 and each strabismic observer is assigned the best-fitting add-on φcrowding, as specified in the legend. Each estimated add-on was adjusted to shift the curves horizontally to match the normal foveal curve. Note that the purely anisometropic amblyope (green) scales with acuity (Panel d) as expected for overlap masking, and the strabismic amblyopes (red and blue) scale with padded eccentricity (Panel f) as expected for crowding.
For grating patches, Latham and Whitaker (1996) showed that the extent of crowding scales linearly with eccentricity, being proportional to eccentricity plus a constant add-on. Figure 3e parallels their finding. (We find the add-on φcrowding to be 0.45° for letters whereas they found it to be roughly 0.2° for gratings.) At every eccentricity, the acuity ratio A'/A is the same function of spacing normalized by padded eccentricity: S/(φ+φcrowding), where φ is eccentricity, and the add-on φcrowding is 0.45°. Like Latham and Whitaker, we find that normalizing spacing by padded eccentricity collapses all the curves into one function. This scaling shows that crowding is similar at all eccentricities, including the fovea. Unlike Latham and Whittaker's round corner, our function (Figure 3e) exhibits a sharp corner, revealing hard limits of size and spacing (dotted and dashed lines). See Appendix B for more discussion of foveal crowding.
One can also think of the patient's central crowding as equivalent to normal peripheral crowding, at some equivalent eccentricity (Table 1). The equivalent eccentricity is
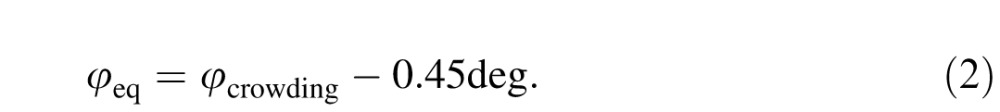 |
Figure 3f shows results for one normal and three amblyopes, all viewing centrally. An eccentricity add-on was assigned to each strabismic amblyope (see legend), sliding the patient's graph horizontally to match that for normal fovea. The collapse of the strabismic amblyopic curves (red and blue) onto the normal foveal curve endorses the eccentricity add-on φcrowding as a good one-number summary of each patient's crowding.
In sum, the purely anisometropic amblyope (green) scales with acuity (Figure 3d) as expected for overlap masking, and the strabismic amblyopes (red and blue) scale with padded eccentricity (Figure 3f) as expected for crowding.
3a. Eccentricity and blur
Normal vision at increased eccentricity has often been suggested as a model for amblyopia, as noted above. Optical defocus (blur) is another appealingly simple model, since the main effect of blur is to impair acuity, and poor acuity is the main diagnostic criterion of amblyopia. The two models are very different. Increasing eccentricity and adding blur have qualitatively different effects on letter identification by normal observers (Figure 4). When viewing directly, adding blur worsens acuity and threshold spacing for letter identification by the same proportion (open circles in Figure 4). In the log-log coordinates, the slope of the regression line (not shown) of thresholds at fixation with blur is 0.99 ± 0.02 (mean ± SE), which is not significantly different from one, so we fit and display a unit-slope line (dotted) log S = 0.14 + log A, i.e., S = 1.4A. In other words, adding blur at fixation (open symbols) increases both spacing and size thresholds by the same proportion, so their ratio S/A is preserved. On the other hand, increasing eccentricity (filled black symbols) results in a disproportionate increase of threshold spacing. This is the well-known crowding effect. The slope of the regression line (dashed) of thresholds at various eccentricities is 1.75 ± 0.17 in the log-log coordinates, telling us that, as eccentricity is increased, threshold spacing increases much faster than acuity. Note that the three filled gray symbols, representing the three normal observers viewing directly, lie along the dotted line for blurred direct viewing, suggesting that this variation among normal individuals reflects differences in their blur (optical and neural).
Figure 4.
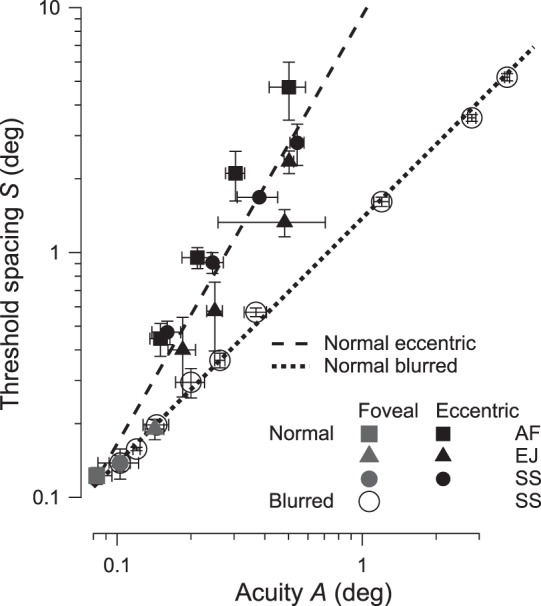
Effects of eccentricity and blur on threshold spacing in normal vision. (The reader is free to think of the vertical scale as 1.1A', instead of S, since S = sA' and the spacing factor s = 1.1.) Filled gray symbols represent the thresholds at fixation, and filled black symbols represent the thresholds at various eccentricities in the periphery of three normal observers. Refractive errors (if any) were fully corrected. Open circles represent the thresholds at fixation of one normal observer wearing blurring lenses of various refractive powers (see Methods). The dashed regression line for the no-blur data at various eccentricities is log S = 0.97 + 1.75 log A, where A is acuity in degrees and S is threshold spacing in degrees. The regression line (not shown) for the zero-eccentricity data at various blurs is log S = 0.13 + (0.99 ± 0.02) log A. Since that slope is insignificantly different from one, we fit and display a line (dotted) with unit slope log S = 0.13 + log A, i.e., S = 1.4A. Note that the three filled gray symbols are all at fixation, so the differences among them are not an effect of eccentricity. They all lie on the blur line (dotted), suggesting that this variation among normal individuals reflects differences in their blur (optical and neural). These two lines, dashed and dotted, are reproduced in several subsequent figures. The letter spacing factor s is 1.1× for all data reported in all tables and figures, except where we indicate otherwise (in Tables 1 and 3 and Figures 2 and 3). The observers are all young normals, so it is surprising that EJ's acuity is so much worse than that of the other two observers. However, AF and SS are emmetropic, while EJ is myopic, −2.50D and −3.00D. Her point lies near the line of optic blur, suggesting that her eyes may have uncorrected optical aberrations.
These two lines show the different effects of eccentricity and blur on normal vision and will be our models for strabismic and purely anisometropic amblyopia. As such, these dotted and dashed lines will reappear in several figures below.
The effect of blur in the normal fovea (dotted line in Figure 4) is remarkable, maintaining perfect proportionality between acuity and threshold spacing over a 40:1 range. What could account for this? If the flankers had no effect, then the flanked acuity would equal the unflanked acuity A' = A and the threshold spacing would be S = sA' = sA. In fact, in these data, the ratio of threshold spacing to acuity S/A is 1.4 ± 0.03, which is much larger than s, which is 1.1. Thus, the flankers are effective over the whole acuity range.
Blur smears the target's image on the retina. The smearing worsens acuity for the target and extends the image of each flanker, which may create enough overlap with the target, at the retina, to produce overlap masking.
3a. Modeling overlap masking
Figure 4 shows that, with blur, the measured threshold spacing was proportional to acuity over a 40:1 range (acuity 0.14° to 5.2°),
 |
where m = 1.4, for identification of a flanked letter on a tight chart (s = 1.1); “m” stands for “masking.”
Overlap masking depends on overlap and decreases rapidly when masker-target spacing is increased beyond contiguity. It is thought to represent interference by the masker's stimulation of the feature detector that normally responds to the target. Presumably there is also some neural “smearing” corresponding to the extension of the neural representation beyond the optical image. We model this by supposing smearing whose extent is a constant fraction μ of the acuity size. When the target and flanker are the same size 𝔸, we suppose that the critical spacing of overlap masking Smasking is the sum of the letter size and the smear,
 |
At this spacing, the spacing factor is s = Smasking/𝔸, so we can plug the letter size 𝔸 = Smasking/s into Equation 4 and solve for Smasking,
 |
This proportionality between spacing and acuity matches the empirical effect of blur (Equation 3), so
 |
Solving for μ, and plugging in the chart spacing s = 1.1 and finding m = 1.4, yields the smear factor,
 |
This is plausible. It is easy to imagine optical and neural smearing extending 13% of the size of an acuity letter. We tested adults with the Sloan font with four flankers at medium contrast. We found the same slope, m = 1.4, and estimate the same smear, μ = 0.13, for blur and anisometropic amblyopia; m (and thus μ) may be larger for children and smaller for less-bold fonts.
3b. Overlap masking versus acuity
The observer's acuity A sets a lower bound on the flanked acuity A'. Overlap masking will raise the flanked acuity A' = S/s above the acuity limit only if Smasking/s > A. Substituting Smasking/A from Equation 5 and solving for s tells us that to detect overlap masking, the chart spacing must be tighter than optimal.
 |
Flanked acuity on a less-than-optimally spaced (s < 1 + μ) chart is determined by overlap masking or crowding, never acuity. Flanked acuity on a more-than-optimally spaced (s > 1 + μ) chart is determined by acuity or crowding, never overlap masking.
Loose, tight, and optimal spacing
Spacings in the range 1.1 ≤ s ≤ 1.4 will be called tight. Larger spacings s > 1.4 will be called loose. The proportionality of overlap masking and acuity (Equation 3) is strongly supported by our data. The dependence of overlap masking on the spacing factor (Equation 5) is speculative, as we tested only one tight spacing, s = 1.1. We expect every tight spacing to perform at least as well as s = 1.1, i.e., to yield a small threshold spacing S ≤ 1.4A, in the absence of crowding. The optimal spacing to detect crowding is the value of s that minimizes threshold spacing in the absence of crowding. That optimum must be in the tight range, and our overlap masking model suggests that it is s = 1 + μ = 1.13.
3c. Strabismic and non-strabismic (purely anisometropic) amblyopia
The amblyopic results (at zero eccentricity with no added blur) are plotted separately for purely anisometropic (Figure 5a) and strabismic amblyopes (Figure 5b). For purely anisometropic amblyopes (Figure 5a), as for the normal observers with added blur, threshold spacing is approximately proportional to acuity. All data points fall near the blurred-normal line (dotted) and far from the eccentric-normal line (dashed). The regression line (solid) for all purely anisometropic amblyopes has a log-log slope of 1.15 ± 0.16, which is not significantly different from 1.0, indicating that purely anisometropic amblyopia is like normal central vision with added blur and unlike normal vision at increased eccentricity.
Figure 5.
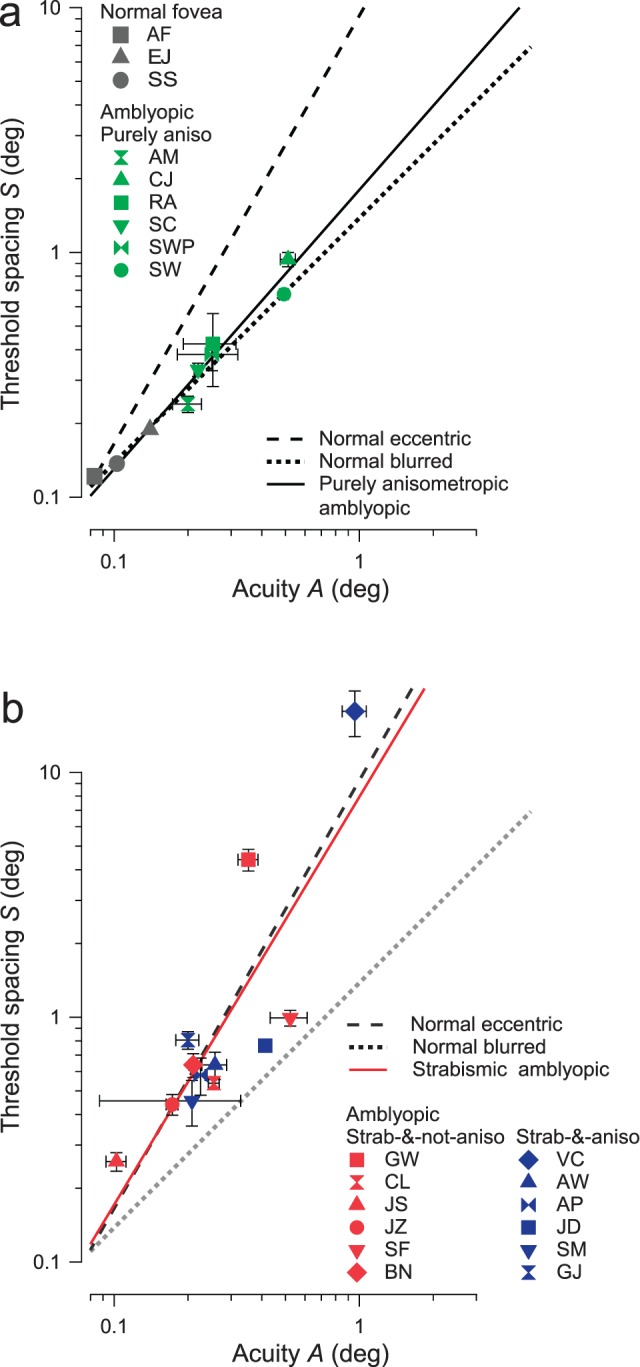
Threshold spacing versus acuity for (a) purely anisometropic (green) amblyopes and (b) strabismic (with or without anisometropia, blue and red) amblyopes. Amblyopic observers' threshold spacings are plotted against acuities. The dashed and the dotted lines are regression lines for normal eccentric and normal blurred results respectively, from Figure 4. The solid lines are the regression lines of (a) purely anisometropic amblyopes' thresholds, log S = 0.26 + 1.15 log A and (b) strabismic amblyopes' thresholds, log S = 0.90 + 1.67 log A. This graph also shows that the combination of threshold spacing and acuity is much better at distinguishing strabismic from purely anisometropic amblyopes than is threshold spacing or acuity alone. In other words, the two diagnostic categories cannot be reliably separated by a horizontal or vertical line, but are well separated by a diagonal line.
Compared to purely anisometropic amblyopes (Figure 5a), the strabismic amblyopes (Figure 5b) show larger variance, but (with two exceptions discussed below) they lie well above the blurred-normal line (dotted) and near the eccentric-normal line (dashed). Since the purely strabismic (red symbols) and strabismic-and-anisometropic results (blue symbols) in Figure 5b are similar, we combined them into one “strabismic” group, regardless of whether anisometropia is an associated condition or not. The regression line (solid) for all strabismic amblyopes has a log-log slope of 1.67 ± 0.17, which is not significantly different from that for normals at increased eccentricities (log-log slope 1.75 ± 0.17).
Figure 5 shows that the amblyopic data are clustered by type, i.e., the strabismic cluster (Figure 5b) has higher threshold spacing than the purely anisometropic cluster (Figure 5a) at any given acuity. Given this arrangement, the two clusters cannot be distinguished by threshold spacing or acuity alone (i.e., a vertical or horizontal line, not shown) but are well distinguished by the combination (e.g., a tilted line, not shown).
Note that the results for one purely strabismic amblyope (SF) and one strabismic-and-anisometropic amblyope (JD) fall close to the blurred-normal regression line (dotted). That may be because they had much more practice. The rest of the observers had done at most a few thousands trials. JD had participated in a host of experiments, including many experiments on crowding, and performed millions of trials with his amblyopic eye. SF had also participated in many experiments, and in particular, he had participated in an intensive training experiment involving roughly 50,000 trials of Vernier acuity, designed to treat adult amblyopes (Li, Klein, & Levi, 2008). As a consequence, his Vernier acuity and his Snellen acuity had both improved substantially. We suspect that this very extensive experience resulted in a reduction in their crowding, similar to the perceptual learning observed in both peripheral (Chung, 2007) and amblyopic vision (Chung, Li, & Levi, 2012; Hussain, Webb, Astle, & McGraw, 2012).
The parameters of each linear regression in the log-log coordinates are listed in Table 2. RMS (root mean square) error is the square root of the mean of the squared differences between the log data and the regression line. The results plotted in Figure 5 and summarized in Table 2 show a qualitative difference between strabismic and purely anisometropic amblyopia in the effect of flankers. The increased-eccentricity model and the added-blur model provide reasonable fits to the threshold spacings and acuities of strabismic and purely anisometropic amblyopes, respectively.
Table 2.
Linear regression of log threshold spacing versus log acuity: log S = a + b log A. Normal blur follows the same line (not significantly different) as purely anisometropic amblyopia. Normal eccentricity follows the same line as strabismic amblyopia. The two lines have substantially different offset a and slope b.
| Normal with various blurs |
Purely anisometropic amblyopes |
Normal at various eccs. |
Strabismic amblyopes |
|
| log S/A ± SE | 0.14 ± 0.01 | 0.18 ± 0.03 | 0.5 ± 0.07 | 0.59 ± 0.11 |
| a ± SE | 0.13 ± 0.02 | 0.26 ± 0.09 | 0.97 ± 0.11 | 0.90 ± 0.11 |
| b ± SE | 0.99 ± 0.02 | 1.15 ± 0.16 | 1.75 ± 0.17 | 1.67 ± 0.17 |
| RMS error | 0.026 | 0.053 | 0.155 | 0.286 |
| Correlation | 0.998 | 0.962 | 0.944 | 0.883 |
Stereopsis was measured using Randot “Random Dot Geometric Form” test (Stereo Optical Co.).
It is clear from Figure 5b that the data from strabismic amblyopes with and without anisometropia (blue and red) have similar distributions. So we can combine all our strabismic amblyopes' data, regardless of anisometropia, into a single group, to get a larger sample of strabismic amblyopes. Beginning with Figure 8, in the rest of our figures (except Figure 11) we do just that, using red to represent strabismic amblyopes with or without anisometropic history and green for purely anisometropic amblyopes.
Figure 8.
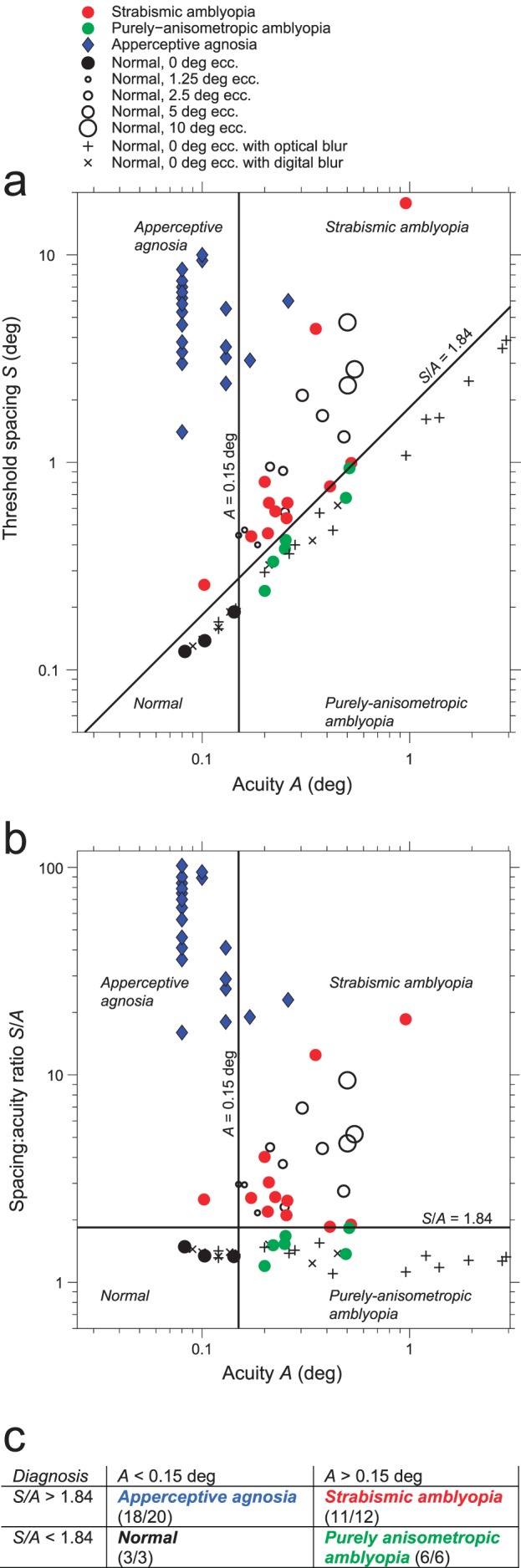
Double dissociation of A and S/A (a) A scatter diagram of threshold spacing S versus acuity A. Normal observers are solid black at ecc. 0° and open circles at eccs. 1.25°, 2.5°, 5°, 10°. Normals with optical or digital blur are + or ×. (0.5 D blur brings normal observer SS to the threshold acuity 0.15°.) Patients are in color. Strabismic amblyopes are red disks. Purely anisometropic amblyopes are green discs. Apperceptive agnosics are blue diamonds. (To prevent occlusion, several agnosics have been shifted up or down ±0.3° along the S axis.) The four clinical groups are quite well separated by the two lines, A = 0.15° and S/A = 1.84. Nearly all (18/20) of the apperceptive agnosics have near-normal acuity, A < 0.15°. Nearly all (11/12) strabismic amblyopes have poor acuity A > 0.15°. (The diagnosis of amblyopia requires impaired acuity, relative to the fellow eye. Strabismic amblyope JS has an acuity of 0.102 in his amblyopic eye, which is normal, but still qualifies as an amblyopic impairment because it's much worse than the unusually good acuity of his fellow eye.) (b) The same lines and data are replotted as spacing:acuity ratio S/A versus acuity A, which makes the dividing lines vertical and horizontal. (c) Summary of the double dissociation. All three normals have good spacing:acuity ratio S/A < 1.84 and acuity A < 1.15. All 20 apperceptive agnosics have high spacing:acuity ratio S/A > 1.84 and nearly all (18/20) have near-normal acuity A < 1.15°. All six purely strabismic amblyopes have impaired acuity A > 1.15° and near-normal spacing:acuity ratio S/A < 1.84. Thus, these two clinical conditions, purely anisometropic amblyopia and apperceptive agnosia, independently affect A and S/A. See Figure 10 for further methodological details of testing the normals and amblyopes.
Figure 11.
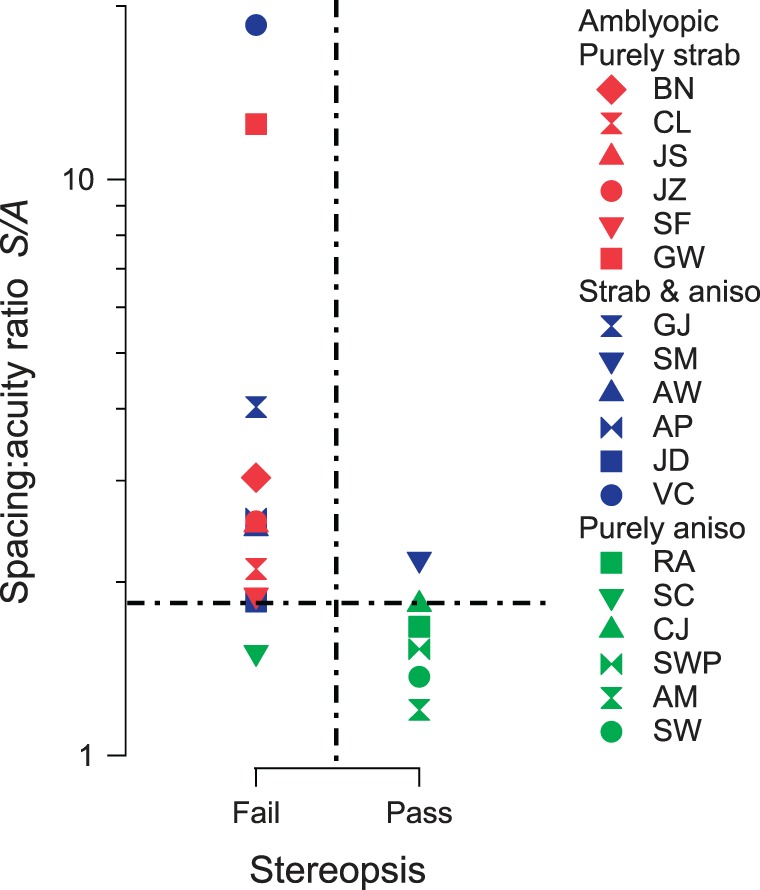
Spacing:acuity ratio versus status of stereopsis for amblyopes. Amblyopes who failed the stereopsis test are plotted as “Fail”; everyone who has some amount of stereopsis is plotted as “Pass.” The vertical dashed line divides the amblyopes into “Fail” and “Pass” groups, and the horizontal dashed line, S/A = 1.84, divides the amblyopes into two groups with large and small spacing:acuity ratio.
3d. Combining blur with eccentricity or amblyopia
For the normal observer, the effect of optical blur on threshold spacing and acuity is strikingly different at fixation (circles in Figure 6) than in the periphery (diamonds and squares). At fixation (circles), blur increases threshold spacing and acuity proportionally, as shown by the unit-slope regression line (dotted) in the log-log coordinates. However, at 5° peripheral, blur still worsens acuity but has much less effect on threshold spacing. As blur grows, the square symbols (near the top) shift substantially rightward (worsened acuity) but very little upward (increased threshold spacing), resulting in a flatter log-log slope. Thus, farther in the periphery, blur still impairs acuity but has less and less effect on flanked acuity. (s is fixed, so flanked acuity is proportional to threshold spacing, A' = S/s.) This is shown more clearly in Figure 6b, which plots the log S versus log A regression line slopes produced by 1 diopter of blur as a function of padded eccentricity (φ + φcrowding). For the normal observer, the slope is one at fixation and falls with eccentricity, nearly reaching zero at 5°.
Figure 6.
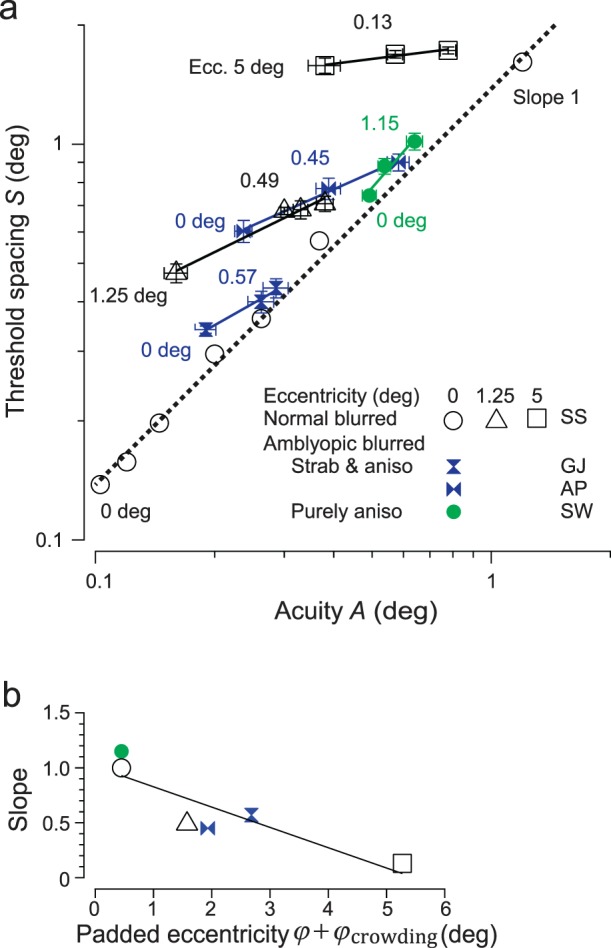
Effect of blur. Threshold spacing is less dependent on acuity (and blur) at greater eccentricity. We measured threshold spacing S versus acuity A for a normal observer (open symbols) and several amblyopes (filled symbols) with various amounts of blur. The normal was tested at several eccentricities; the amblyopes only at fixation. We fit a regression line to each observer at each eccentricity and then plotted the regression line slope (log-log slope of spacing vs. acuity) as a function of padded eccentricity φ + φcrowding. (a) We measured the effect of optical blur. The normal observer (open symbols) was tested at eccentricities of 0° (dotted line), 1.25° (open triangle), and 5° (open square). Amblyopes (filled symbols) were tested at fixation (0°). Each measurement was obtained while the observer was wearing a blurring lens (see Methods) except for the leftmost data point for each observer, which was measured merely with the observer's refractive correction. The dotted regression line is a unit-slope line for a normal observer viewing directly with blur, from Figure 4. A regression is shown for the blur results (with a range of at least one diopter) for each observer and eccentricity tested (see Methods). (b) The lower graph plots the slope of each regression, as a function of the padded eccentricity (φ + φcrowding). For the normal observer, the add-on is fixed φcrowding = 0.45°, and several eccentricities φ are tested. For the amblyopes, eccentricity is zero φ = 0°, and we used the observer's add-on φcrowding from Table 1. Regression lines. (a) SS at 1.25°: log S = 0.07 + 0.49 log A; SS at 5°: log S = 0.25 + 0.13 log A; GJ: log S = 0.06 + 0.57 log A; and AP: log S = 0.06 + 0.45 log A. (b) Regression line is: Slope = 1.0 – 0.19 (φ + φcrowding).
The amblyopes were all tested at fixation, but each amblyope has a different “equivalent eccentricity” φeq at which a normal eye has the same crowding as this amblyopic fovea (Table 1). Figure 6b accommodates this by using the appropriate add-on φcrowding = φeq + 0.45°. The normal and amblyopes all show the same trend. The log-log slope is about one at fixation and drops with increasing padded eccentricity.
This makes sense. Critical spacing is determined by whichever is worse, overlap masking or crowding. At low padded eccentricity, critical spacing is determined by overlap masking. The critical spacing of crowding grows proportionally with padded eccentricity and eventually dominates. Overlap masking depends on acuity, and crowding does not.
3e. Is amblyopia like optical blur, subjectively?
Since blur is a good model for the effect of purely anisometropic amblyopia on legibility it is interesting to ask whether blurring the good eye gives the same subjective experience as vision through the amblyopic eye. Irvine (1945) noted, “If the good eye is blurred to a visual acuity equal to that of the amblyopic eye the contrast [i.e. the difference between eyes] to the patient is obvious, i.e., to the good eye the minimal visible letter is blurred and hazy, whereas to the amblyopic eye it is black, easily seen but is uninterpretable.” We asked our amblyopic observers to compare the suprathreshold appearance of our target letters seen through one eye or the other, and their reports confirm Irvine's observation: High contrast letters do not appear faint or blurred when viewed with the amblyopic eye. Our high contrast (90%) stimuli are perceived to have equal contrast in the two eyes of both strabismic and purely anisometropic amblyopes. This nearly normal suprathreshold contrast perception has been previously reported in amblyopia (Hess & Bradley, 1980; Loshin & Levi, 1983). High-contrast stimuli are perceived as high contrast in both strabismic and purely anisometropic amblyopes.
We also confirmed that briefly blurring the nonamblyopic eye to the same visual acuity as that of the amblyopic eye does not mimic the perception of amblyopia. Both strabismic and purely anisometropic amblyopes report that letters look blurrier when viewed with the blurred nonamblyopic eye than with the amblyopic eye. However, these experiments were brief. With more exposure time, minutes or hours, people adapt to image blur (Elliott, Georgeson, & Webster, 2011; Georgeson & Sullivan, 1975). After adapting to chronic blur, images no longer look hazy or blurry. Thus, the perceptual effect of anisometropic amblyopia seems to be like that of chronic, not temporary, optical blur. Thus, it seems that chronic blur would be a good model for both the objective and subjective aspects of purely anisometropic amblyopia.
3f. Equivalent blur model for anisometropic amblyopia
We define the equivalent blur of a given acuity as the amount of defocus for a normal observer, which yields that acuity. As shown in Figure 7, a simple model A = k
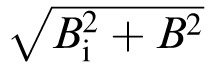 describes the normal relationship between acuity and defocus, where A is acuity, k is a proportionality constant, Bi is the intrinsic blur, and B is the external blur (defocus, in diopters) added to the test eye (Levi & Klein, 1990a, 1990b; Watt & Hess, 1987; Watt & Morgan, 1984). The inverse of this dependence of acuity on optical blur is our equivalent-blur model of purely anisometropic amblyopia.
describes the normal relationship between acuity and defocus, where A is acuity, k is a proportionality constant, Bi is the intrinsic blur, and B is the external blur (defocus, in diopters) added to the test eye (Levi & Klein, 1990a, 1990b; Watt & Hess, 1987; Watt & Morgan, 1984). The inverse of this dependence of acuity on optical blur is our equivalent-blur model of purely anisometropic amblyopia.
Figure 7.
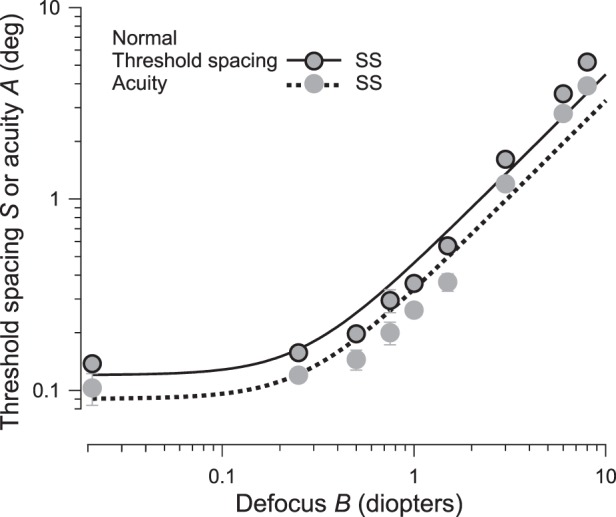
Acuity and threshold spacing versus defocus in normal central vision. The gray disks, with or without a black edge, represent the threshold spacing or acuity, respectively. The fitted curve (solid) for threshold spacing is S = 0.45
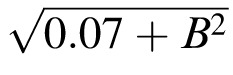 ; the fitted curve (dotted) for acuity is A = 0.33
; the fitted curve (dotted) for acuity is A = 0.33
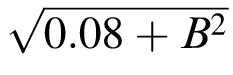 , where B is defocus in diopters. The inverse of the dotted curve for acuity is our equivalent-blur model of purely anisometropic amblyopia. The spacing:acuity ratio S/A = 1.4 ± 0.03 is not significantly different from m = 1.4, indicating that the threshold spacing is limited by overlap masking.
, where B is defocus in diopters. The inverse of the dotted curve for acuity is our equivalent-blur model of purely anisometropic amblyopia. The spacing:acuity ratio S/A = 1.4 ± 0.03 is not significantly different from m = 1.4, indicating that the threshold spacing is limited by overlap masking.
4. Spacing:acuity ratio S/A
Up to this point, we have considered spacing and acuity separately; however, we will see below that the ratio of threshold spacing to acuity S/A perfectly separates purely anisometropics from strabismics in our 18 amblyopes (Figure 11).
5a. Apperceptive agnosia
Apperceptive agnosia is a disorder in visual object recognition that cannot be attributed to visual field loss, impaired acuity, language, or memory, or general deterioration (De Renzi, 1996). Strappini et al. (submitted) provided us with measured acuity and estimated equivalent eccentricity of crowding for 20 patients diagnosed with apperceptive agnosia. These are shown as blue diamonds in Figures 8, 9, and 12. Note that over half of the apperceptive agnosics (12/20) have exactly the same acuity, 0.08°. This is because neurology clinics are not interested in the normal range of acuity, so they stop testing once the patient achieves an acuity of 0.08°. Each patient was tested with several object recognition tasks, from which Strappini et al. estimated their equivalent eccentricity of crowding, from which we used Equations 2 and 13 to estimate threshold spacing S = Scrowding.
Figure 9.
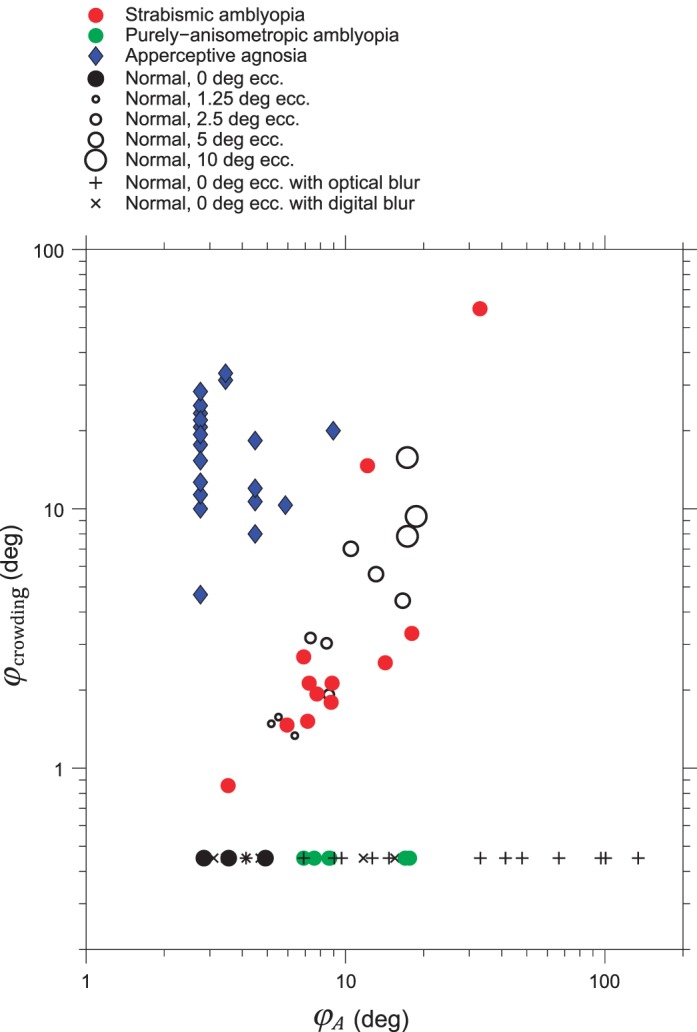
Characterizing each observer and condition by the two add-ons, φA and φcrowding. This plots estimated parameters (φcrowding and φA from Equations 15 and 16) instead of the raw data (S and A). This makes the diagnostic categories more obvious than in the raw plot of Figure 8a. Here, S and A are measured at fixation, φ = 0. φA is proportional to A, but φcrowding is nonlinearly related to S and A. At high S/A, φcrowding is proportional to S, but at low S/A, φcrowding is constant. The nonlinearity pushes all the normal, blur, and purely anisometropic points down to the floor. This analysis and presentation show that purely anisometropic amblyopia (and blur) affect only φA, not φcrowding; strabismic amblyopia impairs both φA and φcrowding; and apperceptive agnosia greatly impairs φcrowding and typically spares φA.
Figure 12.
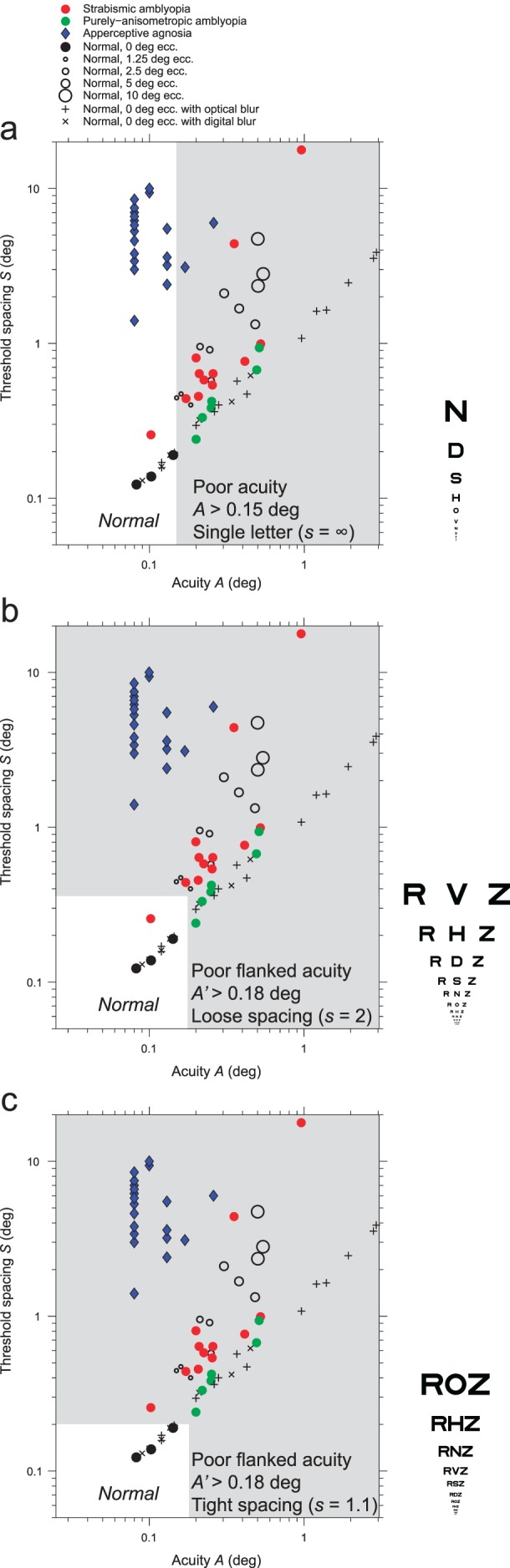
The pass/fail regions for screening by (a) single-letter acuity or flanked acuity with (b) loose or (c) tight spacing. (Data from Figure 8a.) The single-letter acuity test passes anyone who reads 0.15° letters. Thus, it passes the normals, and detects most of the patients, but fails to detect the patients who have normal acuity. The flanked acuity tests are more demanding. We relaxed the size criterion slightly, to 0.18°, but observers must read the 0.18° letters at 0.36° spacing on the loose chart (b) or 0.2° spacing on the tight chart (c). Flanked acuity passes all three normals. With loose spacing (b) it detects all but one of the patients (a strabismic amblyope). With tight spacing (c) it detects all the patients.
The 20 patient results provided by Strappini et al. (submitted) are their analyses of published reports: two patients (P1, P2) from Crutch and Warrington (2007), two (CRO, SCI) from Crutch and Warrington (2009), and one patient from each of the following papers: Behrmann and Kimchi (2003); Behrmann, Moscovitch, and Winocur (1994); Behrmann and Williams (2007); Boucart et al. (2010); Buxbaum et al. (1999); Delvénne, Seron, Coyette, and Rossion (2004); Foulsham, Barton, Kingstone, Dewhurst, and Underwood (2009); Funnell and Wilding (2011); Gilaie-Dotan, Perry, Bonneh, Malach, and Bentin (2009); Giovagnoli et al. (2009); Hiraoka, Suzuki, Hirayama, and Mori (2009); Jankowiak, Kinsbourne, Shalev, and Bachman (1992); Joubert et al. (2003); Leek, Patterson, Paul, Rafal, and Cristino (2012); Mendez, Shapira, and Clark (2007); Riddoch and Humphreys (1987). Each patient's equivalent eccentricity was estimated from performance of more than one of the following object recognition tests: Double Letters Identification, Crowding Test, Crowding Test Different Flankers, Crowding Test Similar Flankers, Snodgrass & Vanderwart, Boston Naming Test, Birmingham Object Recognition Battery (BORB) (single features). Acuity was measured with single letters or symbols (s = ∞), mostly with a subtest of the Cortical Vision Screening Test (CORVIST) (Warrington, Plant, & James, 2001). Please see the forthcoming paper by Strappini et al. for the inclusion criteria and other details.
5b. Double dissociation of A and S/A
It has often been assumed that acuity A and critical spacing S are tightly linked, but this is only true for overlap masking, not for crowding. In concert with Strappini et al. (submitted), we jointly report a double dissociation. Two clinical conditions independently affect acuity A and the spacing:acuity ratio S/A. Purely anisometropic amblyopia (like optical blur) impairs acuity A without affecting S/A. Apperceptive agnosia greatly increases S/A while hardly affecting acuity A (Strappini et al., submitted). Applying cut offs to these two parameters (Figures 8a, b) sorts the observers into four diagnostic groups (Figures 8b, c). Models of human object recognition will need to accommodate this independence of acuity and the spacing:acuity ratio.
The diagnostic value of the spacing:acuity ratio in Figure 8 is new. The success of acuity is expected because it is built into the definitions. The diagnosis of amblyopia requires an acuity deficit (albeit relative to the other eye, which is usually normal), and the diagnosis of apperceptive agnosia requires near normal acuity.
5c. Double dissociation of acuity and crowding
Figure 8a shows that the four diagnostic categories (normal, purely anisometropic amblyopia, strabismic amblyopia, apperceptive agnosia) are linearly separable in the raw data. (The axes S and A are proportional to the measured size thresholds A' and A.) Figure 8b shows a double dissociation between S/A and A. That is nice, but S/A does not seem fundamental to us, and there is a better, simpler, way of thinking about this. Across disease categories, crowding may be independent of acuity, but overlap masking is proportional to acuity, Smasking = 1.4A (Equation 5, s = 1.1, μ = 0.13). Spacing thresholds for anisometropic amblyopes or blur will include overlap masking and its tightly linked spacing and acuity. To reveal the independence of crowding from acuity, it is necessary to include only the threshold spacings that are due to crowding, omitting those that are due to overlap masking. Equation 15 makes that selection to estimate the observer's eccentricity add-on for crowding. From the (S,A) data in Figure 8, we used Equations 15 and 16 to estimate each observer's crowding and acuity parameters φcrowding and φA and plotted them in Figure 9.
Figure 9 plots the result, revealing a double dissociation between crowding and acuity. Purely anisometropic amblyopia (or blur) greatly impairs acuity without affecting crowding. Apperceptive agnosia greatly worsens crowding with hardly any effect on acuity. Strabismic amblyopia (or increased eccentricity) impairs both.
6 & 7. (Not present in this section.)
Discussion
The subsection numbering here corresponds to the numbering of the final Summary and conclusions section.
First (1,2), we present a simple model for legibility of a flanked letter. It accounts for all our results in terms of size and spacing limits due to acuity, overlap masking, and crowding. Next (3), we show that strabismic amblyopia is much like normal vision at increased eccentricity and that purely anisometropic amblyopia is much like normal vision with added blur. Then (4), we show that flanked and unflanked acuity, together, accurately distinguish strabismic from purely anisometropic amblyopia, although this is not possible with either measure alone. Our results are mostly for brief medium-contrast white letters, but in Appendix A we extend our conclusions to a wide range of viewing conditions, including static high-contrast black letters, the mainstay of clinical testing. Finally (7), we discuss implications for visual screening. Simple changes to existing tests may substantially increase their sensitivity without reducing their specificity.
1. Size and spacing
First, we recap our results. For normal and amblyopic observers at several eccentricities, a systematic exploration of the size and spacing requirements for identification of a flanked letter reveals that all the thresholds are close to whichever limit, size or spacing, is more severe. Thus the two limits, size and spacing, together account for all the data (Figures 2 and 3). Moreover, we can identify the failure mechanism responsible for each limit. We consider acuity, crowding, and overlap masking. In Figures 2 and 3, the normal data are on the left and the amblyopic data are on the right.
Acuity
The size limit (dashed line) in Figure 2 is acuity. The vertical position of the horizontal dashed line in Figure 2a, b is fitted to go through the measured acuity (the point at infinite spacing). Thus acuity, represented by the dashed lines, accounts for the data at very loose spacing where flankers have no measureable effect.
Crowding
In principle, the spacing limit (dotted line) could be due to crowding or overlap masking. The critical spacing of crowding is known to be linearly related to eccentricity with a proportionality of b, the Bouma factor, of “roughly half” (Bouma, 1970; Pelli & Tillman, 2008). Indeed the spacing limit for the normal at every eccentricity corresponds to b = 0.3 (dashed line Figure 3e). Note that the horizontal scale is S/(φ + φcrowding), and b is defined as b = Scrowding/(φ + φcrowding). Thus, at every eccentricity, including fixation, normal thresholds at tighter spacings (s < 1 + μ = 1.13) are accounted for by crowding (dashed line) and normal thresholds at looser spacings (s > 1 + μ = 1.13) are accounted for by acuity (dotted line).
Assigning an equivalent eccentricity (φeq = φcrowding-0.45°) to each strabismic amblyope aligns them with the b = 0.3 spacing limit (Figure 3f). We attribute the strabismic amblyopes' spacing limit to crowding, with abnormally high φcrowding (Equation 13). We attribute the purely anisometric amblyope's spacing limit to overlap masking (Equation 5).
Overlap masking
The retinal image is an optically blurred image of the display, so the target and flankers extend farther and overlap more in the retinal image than at the display. As noted in Results, the proximity of the target and flankers (e.g., spacing factor of 1.1) makes overlap masking a plausible mechanism. We expect overlap masking to limit flanked acuity only on tighter charts (s < 1.13). We find that the neural deficit of purely anisometropic amblyopia is like optical blur, so the effective blur or “smearing” in normals and amblyopes may be a result of both optical and neural blur. We suppose that the extent of smearing is a fixed fraction of the acuity size, so we expect the critical spacing of overlap masking to be proportional to acuity (Equations 3 and 5). This prediction is borne out beautifully in the normal blur data of Figure 4. The ratio m of threshold spacing to acuity is almost exactly 1.4 over a 40:1 range of size (0.14° to 5.2°). Applying this spacing limit S ≥ Smasking = 1.4A to the S versus s data in Figure 2, we find that it can account for only the lower leftmost point in Figure 2a, c. (This is a surprising coincidence since we saw above that this point is also well accounted for by crowding, as discussed in Appendix B.) Thus, for our normal data, overlap masking may account for, at most, the threshold spacing at fixation when the spacing is tighter than optimal (s < 1.13). The rest of the normal data (s > 1.13) are accounted for by crowding or acuity, as noted above.
A model for legibility of a flanked letter
The lines in Figure 2 represent a simple model for the size and spacing requirements of letter identification. There are three size-and-spacing limits to legibility. A flanked letter is legible only if it respects all three limits. The acuity limit is that the flanked letter size must be at least acuity A, so the spacing will be at least Sacuity = sA. The crowding limit is that spacing must be at least the critical spacing of crowding Scrowding. The overlap-masking limit is Equation 5. Thus, by expanding Scritical = max (Scrowding, Smasking) in Equation 1, we get our model for threshold spacing S on a flanked acuity chart,
 |
Our model of overlap masking (Equation 5) on a more-than-optimally spaced chart (s > 1 + μ) never exceeds the acuity limit, Smasking < Sacuity, and on a less-than-optimally spaced chart (s < 1 + μ) always exceeds the acuity limit Smasking > Sacuity. This splits Equation 9 into two cases, of high and low s,
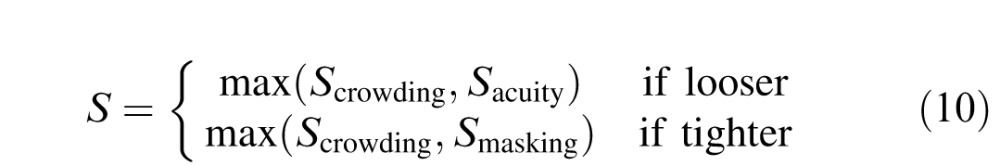 |
Plugging in the definitions of Sacuity and Smasking makes the prediction explicit.
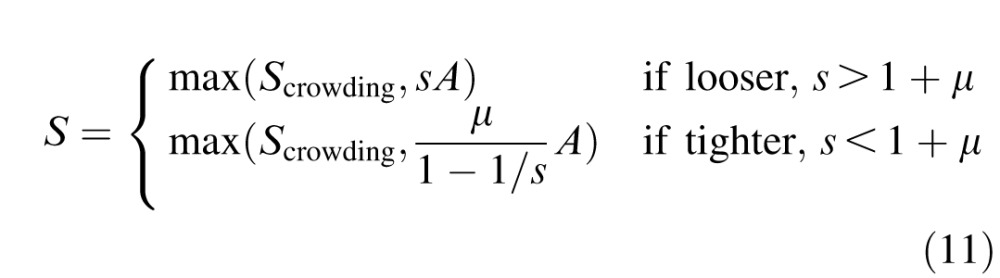 |
The diagnostic power of this test in detecting patients with crowding among normals grows with the difference in threshold spacing, Scrowding- max(Smasking, Sacuity). Our data show that, unlike acuity and overlap masking, the crowding limit Scrowding is hardly dependent on optical blur and contrast. It seems to be a fundamental parameter of vision. Scrowding is independent of the chart spacing factor s, but Smasking and Sacuity both depend on it. Thus the diagnostic power of the chart to detect crowding is maximized by choosing the chart spacing s that minimizes the threshold spacing in the absence of crowding, max(Sacuity, Smasking). The minimum of max(Sacuity, Smasking) = max(s, μ/(1 − 1/s))A is at s = 1 + μ = 1.13, but we used a slightly tighter spacing of s = 1.1. Plugging in our estimate of smear μ = 0.13 and our tighter than optimal spacing s = 1.1 reduces our model down to this:
 |
Equation 12 also applies to amblyopes. The diagnosis of amblyopia requires an acuity deficit relative to the other eye, so most amblyopes have abnormally high A. We find that strabismic amblyopes always have abnormally high Scrowding, and that non-strabismic amblyopes never do. Under our testing conditions, for the purely anisometropic amblyope (Figure 2bd, green), as for the normal, only the data point with tight spacing (s = 1.1) at fixation is accounted for by overlap masking. Increasing the spacing from tight (s = 1.1) to loose (s = 1.6) switches the spacing limit from overlap masking 1.4A to acuity sA = 1.6A. Using the tight spacing, we find a fixed spacing:acuity ratio S/A = 1.4 ± 0.03 for the normal over a wide range of optical blur (Figure 4) and practically the same ratio 1.5 ± 0.09 for the purely anisometropic amblyopes without added blur (Figure 5a).
The generality of our result
We tested normal and amblyopic adult observers. Most of our data are for briefly presented moderate to high contrast white letters. As reported in Appendix A, we have also explored a wide range of durations (13 ms to unlimited), contrast (0.15 to 0.9), and both contrast polarities. In brief, measuring acuity and flanked acuity with a tight spacing provides a screening test for strabismic amblyopia in adults that is robust across stimulus conditions.
2. Linear dependence on eccentricity
It is well known that acuity A and the critical spacing of crowding Scrowding increase linearly with eccentricity. Thus we can extend the legibility model to all eccentricities by expressing the acuity and spacing limits as linear functions of eccentricity (Levi, Klein, & Aitsebaomo, 1985; Toet & Levi, 1992),
 |
 |
where b is the Bouma factor. Equation 13 shows that crowding is linearly related to eccentricity φ and proportional to “padded” eccentricity φ + φcrowding. So padded eccentricity is a good scale against which to examine effects of crowding. For normal observers identifying print-like letters, we estimate the parameter values to be φcrowding = 0.45°, b = 0.3, φA = 2.72°, and a = 0.029.
In patients who, like amblyopes, are nearly normal in the far periphery, the proportionality constants a and b must be practically normal, so they fully specified once we estimate their eccentricity add-ons for acuity and crowding: φA and φcrowding. We now solve what we have to obtain formulas to compute the eccentricity add-ons from measurements at fixation. Solving Equation 14 for φA yields Equation 16. Solving Equation 11 for Scrowding tells us that Scrowding = S if the threshold spacing is due to crowding, and that Scrowding is unknown, no larger than S, otherwise. When Scrowding is known, we can solve Equation 13 for φcrowding. When Scrowding is unknown, no larger than S, our best guess for φcrowding is its normal value, 0.45°,
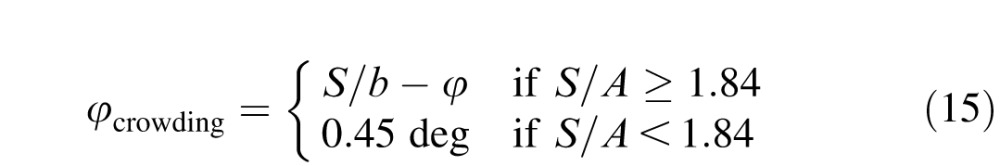 |
 |
where S and A are measured at eccentricity φ, and a and b are the proportionality constants in Equations 13 and 14. The critical value of 1.84 was found for a tight chart (s = 1.1). For a loose chart, the optimal value of the criterion might be slightly higher than 1.84.
In typical conditions, including normal, refractive error, and amblyopia, the threshold spacing and acuity depend linearly on eccentricity (Equations 13 and 14) with known proportionality constants a and b. Then, measuring just flanked and unflanked acuity, to get S and A, suffices to estimate the two eccentricity add-ons φcrowding and φA (Equations 15 and 16). That is enough for our model (Equations 13 and 14) to predict the observer's threshold size and spacing for letters at any eccentricity.
Equations 13 and 14 extend our model to all eccentricities. Each observer can be characterized by two numbers, the eccentricity add-ons for acuity and critical spacing. These two numbers characterize the limits of letter recognition in normal and patient populations across the visual field. Surprisingly, they are not tightly linked, and, in fact, we will show a double dissociation below.
Substituting Equations 13 and 14 into the legibility model (Equation 11) shows the model's eccentricity dependence
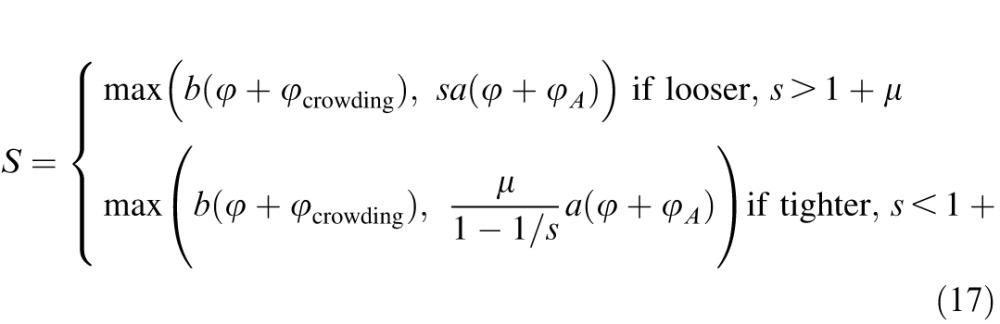 |
which reduces to
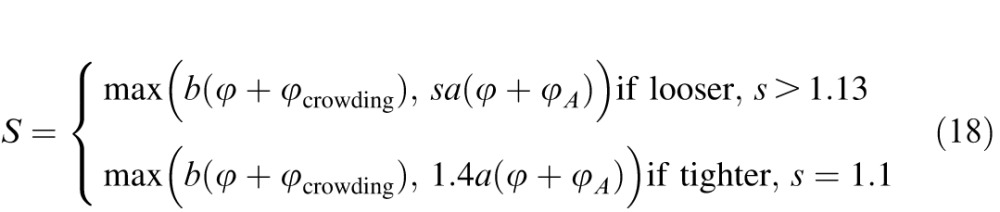 |
In the far periphery (φ > 4°), amblyopes have nearly normal acuity and critical spacing (Levi, Song, & Pelli, 2007), which indicates that they have normal values for the proportionality constants a and b in Equations 13 and 14. Thus, as letter identifiers, amblyopes differ from normal solely by having larger eccentricity add-ons φcrowding and φA.
Recommendation
To characterize a patient, we suggest measuring flanked and unflanked acuity, A' and A, to get acuity A and threshold spacing S = sA', and then using Equations 15 and 16 to estimate the crowding and acuity add-ons φcrowding and φA. With those two numbers, Equations 14 and 18 predict the observer's acuity and threshold spacing over the entire visual field.
Crowding in the fovea
There has been some controversy as to whether there is crowding in the normal fovea (see for example Coates & Levi, 2014; Siderov, Waugh, & Bedell, 2013). We find support for both sides. Regarding crowding, Figure 3 and Appendix B show that the critical spacing of small targets in the fovea is in keeping with the crowding measured peripherally. Regarding overlap masking, Figures 2 and 3 show that the spacing:acuity ratio S/A at fixation for our normal observers was about 1.4, which is the hallmark of overlap masking (as shown in Figure 4). Figure 16 in Appendix B shows that foveal flanked acuity is unchanged when crowding is abolished. It seems that the critical spacing in the fovea is equal for crowding and overlap spacing.
Crowding and reading
The critical spacing for reading is equal to the critical spacing for crowding (Levi, Song, & Pelli, 2007; Pelli & Tillman, 2008; Pelli et al., 2007). When text is more closely spaced than critical, reading slows to a crawl. Critical spacing is very much larger in the periphery and in strabismic amblyopes. The maximal reading rate is greatly reduced in the periphery but is unaffected by amblyopia. This surprising result—no effect of amblyopia in the periphery—is predicted by the uncrowded-span model (Pelli et al., 2007), given the observation that the amblyopic crowding deficit is strictly central. Amblyopia greatly increases the small critical spacing (flanked letter acuity) in the central visual field, without affecting the larger critical spacings found more peripherally (Levi, Song, & Pelli, 2007), which determine the uncrowded span and thus reading rate.
3. Equivalent eccentricity and blur
Strabismic amblyopia is like increased eccentricity
A number of studies have reported large flanker effects in strabismic amblyopes, even in those who have only mildly impaired unflanked visual acuity (Bonneh et al., 2004; Hess & Jacobs, 1979; Levi, Hariharan, et al., 2002a). In the current study, superimposing the regression lines for normal vision (from Figure 4) on Figure 5b shows that the strabismic amblyopes' regression line (solid) lies above the blurred-normal regression line (dotted), i.e., for any given acuity, strabismic amblyopes have larger threshold spacing than do the normals with added blur. Thus, strabismic amblyopia is unlike blur. Instead, the strabismic-amblyope results are reasonably well described by the regression line for normal observers at increased eccentricities (dashed). We therefore hypothesize that the threshold spacing for strabismic amblyopia is mediated by crowding, as in normal peripheral vision. If our hypothesis—that strabismic amblyopia is like increased eccentricity—is correct, then adding optical blur to strabismic amblyopes at fixation should result in a similar relationship between the threshold spacing and acuity as that for normals at increased eccentricity. Indeed, Figure 6 shows that the threshold spacing increases much more slowly than the acuity does (log-log slope <1) when optical blur is added at fixation for strabismic amblyopes, as in normal peripheral vision.
Purely anisometropic amblyopia is like blur
Purely anisometropic amblyopes show a proportional relationship between threshold spacing and acuity, and their data closely match the blurred-normal regression line for direct viewing (Figure 5a). We find that the threshold spacing for purely anisometropic-amblyopic central vision can be reproduced in the normal simply by scaling the acuity size of the stimuli. This suggests that a common mechanism, namely overlap masking, is responsible for the threshold spacing at fixation in both normal and purely anisometropic-amblyopic observers. (Appendix B says more about what limits critical spacing at fixation.) Moreover, at fixation, adding optical blur to purely anisometropic amblyopes results in a proportional increase in threshold spacing and acuity. This proportionality is also seen in the results for normal direct viewing with blur (dotted line in Figures 4 and 5). In fact, scaling effects have been previously reported in other visual functions for purely anisometropic amblyopia. For example, comparing Vernier acuity and visual resolution reveals a linear relationship between them (Levi & Klein, 1982, 1985). Another example is that the abnormal contour integration for the amblyopic eye of purely anisometropic amblyopes becomes nearly normal once the target visibility is equated between the amblyopic and nonamblyopic eyes, i.e., the contrast and spatial scale of the stimuli are adjusted for the amblyopic eye such that its performance in detection equals that of the nonamblyopic eye (Hess & Demanins, 1998). Consistent with our results, Bonneh et al. (2004) found that the flanked letter acuity is better correlated with the unflanked letter acuity for purely anisometropic amblyopia than for strabismic amblyopia.
Eccentricity trumps blur
Eccentricity and blur are powerful manipulations. Each models one kind of amblyopia. What happens when they are combined? Figure 6 shows that adding optical blur at increased eccentricity (instead of at fixation) results in a disproportionately small increase in the threshold spacing relative to the acuity. Threshold spacing is hardly affected by blur in the periphery but strongly affected at fixation. Hence, the optical resolution of the eye does not contribute significantly to the flanker effect at large eccentricities. In other words, it is eccentricity, not acuity, that determines the peripheral flanker effect. This shows that crowding, rather than overlap masking, determines the threshold spacing at increased eccentricity. Consistent with our conclusion, many studies have shown that the critical spacing in the periphery is determined by crowding, which is size independent and eccentricity dependent (Chung, Levi, & Legge, 2001; Intriligator & Cavanagh, 2001; Levi, Hariharan, et al., 2002b; Pelli et al., 2004; Strasburger, 2005). See Pelli and Tillman (2008) and Whitney and Levi (2011) for reviews.
Null hypotheses
The simplest explanation for the success of the equivalent-blur and equivalent-eccentricity models would be that the models are literally true. Perhaps strabismic amblyopic eyes fixate poorly and thus view stimuli eccentrically, and purely anisometropic-amblyopic eyes focus poorly (or have uncorrected high-order aberrations) and thus have greater optical blur than eyes with normal vision. We did further experiments and analyses to test (and reject) these null hypotheses (Appendix C).
4. Spacing:acuity ratio S/A
Figure 10 plots the spacing:acuity ratio S/A against unflanked acuity A for all the conditions that we tested, spanning a wide range of contrast, duration, polarity, pupil size (with and without 2 mm pinhole), and eccentricity (0° and 5°) for normal observers and amblyopes. The horizontal line at S/A = 1.84 (dashed) distinguishes strabismic amblyopia (red) from anisometropic amblyopia (green). For normal observers, all the peripheral points (circles) are above this criterion, and all the foveal points (black) are below. Figure 10 supports our finding that eccentricity (circles) models strabismic amblyopia (red) and that blur (+ and ×) models purely anisometropic amblyopia.
Figure 10.
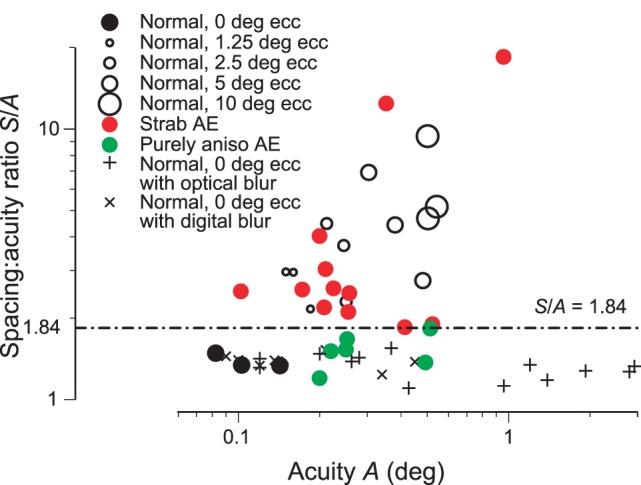
The boundary between crowded and uncrowded. (These data also appear in Figure 8.) Spacing:acuity ratio versus acuity for normal and amblyopic observers. White letters are displayed on a CRT at medium contrast (0.6) and 1.1× spacing for 200 ms. (See Figure 15 in Appendix A for more conditions.) The observers are normals (black symbols) or strabismic (red) or purely anisometropic (green) amblyopes. Normals are tested at eccentricity zero (filled black circles) and 1.25°, 2.5°, 5°, and 10° (circles, small to large). The symbols + and × represent results with optical and digital blur, respectively. All but one of the strabismic-amblyope and normal peripheral ratios are above the 1.84 criterion (dot-dashed line). All of the purely anisometropic-amblyope and normal central ratios, including those with optical and digital blur, are below the 1.84 criterion. Thus, all the conditions limited by crowding are above the line, and the rest are below the line.
We find, in close agreement with Bonneh et al. (2004), that the effect of flankers on acuity is qualitatively different in strabismic and purely anisometropic amblyopes. The difference between strabismic and purely anisometropic amblyopia is evident in the combination of flanked and unflanked acuity but not in either alone (Figure 5).
The spacing:acuity ratio S/A is the ratio of threshold spacing S to acuity A. Thus S/A = sA'/A. Given a spacing factor s, the spacing:acuity ratio is proportional to the “crowding ratio” or “crowding index” A'/A used by Atkinson et al. (1988) and Levi, Yu, Kuai, and Rislove (2007). The spacing:acuity ratio tells us about the relative importance of spacing (due to crowding or overlap masking) and size (due to acuity). S/A equals s when flanked legibility is limited by size (A' = A), and S/A exceeds s when flanked legibility is limited by spacing (A' > A).
Vernier acuity
The spacing:acuity ratio introduced here behaves much like one over the resolution/Vernier ratio of the older literature. Both ratios are normal in purely anisometropic amblyopes and abnormal in strabismic amblyopes. Indeed the add-on eccentricity (or “E2”) for the critical spacing of crowding (0.45°) is consistent with the estimated E2 value (0.3° to 0.9°) for Vernier acuity, bisection, and spatial interval discrimination (Levi & Carkeet, 1993). This indicates that Vernier acuity is proportional to the critical spacing of crowding. The impairment of Vernier acuity in the normal periphery and strabismic amblyopia has been attributed to “spatial uncertainty.” We suggest that the “spatial uncertainty” is crowding.
Stereopsis
A large-scale study of amblyopia found that reduced visual resolution and loss of binocularity are key in determining the pattern of visual deficits (McKee et al., 2003).
Figure 11 and Table 1 show that all but one of the strabismic amblyopes (with or without anisometropia) failed the stereopsis test, whereas all but one of the purely anisometropic amblyopes have some stereopsis. Indeed, the one strabismic amblyope who “passed” the stereopsis test had considerably poorer stereoacuity than the five purely anisometropics who passed. Figure 11 plots the spacing:acuity ratio, which is an indicator of crowding, against the status of stereopsis of each amblyopic observer. The two measures, stereopsis and the spacing:acuity ratio, are both practically perfect in classifying the kind of amblyopia (strabismic vs. purely anisometropic) of our 18 amblyopes. The spacing: acuity ratio test makes no mistake (Figure 11); the stereopsis test misclassifies two of the 18 amblyopes (lower left and upper right).
In practice, clinicians can use the spacing:acuity ratio to distinguish the two kinds of amblyopia. For theory, the high correlation between binocularity (stereopsis) and crowding (critical spacing) suggests a developmental link.
Three diagnostic indicators
Thus, among our 18 amblyopes, we find near-perfect agreement among three diagnostic indicators: history of strabismus, absence of stereopsis, and a high spacing:acuity ratio (S/A > 1.84). Note that the cut-off points for these binary classifications were conventional and predetermined for strabismus and stereopsis, but the 1.84 cut off for the spacing:acuity ratio was chosen after the fact, to best agree with the strabismus indicator. Also note that the value of S/A depends on the tightness of the spacing s. Below we recommend testing with optimal spacing (for maximum sensitivity to crowding) when screening patients to determine who needs further professional attention.
5 & 6. (Not present in this section.) : 7a. Screening
Population screening trials have demonstrated a very favorable cost-benefit ratio in screening children for amblyopia (Atkinson et al., 2007; Clarke et al., 2003; Joish et al., 2003; but see Snowdon & Stewart-Brown, 1997). It is well established that acuity for “surrounded” optotypes (i.e., flanked acuity) is much more sensitive to strabismic amblyopia than is acuity for “isolated” optotypes (unflanked acuity), and the test designers largely heed this (Ehrlich, Reinecke, & Simons, 1983; Thomas-Decortis, 1959). Flanked acuity is tested by charts in which the other letters act as flankers or by displaying a single target letter surrounded by target-like flankers, as in Figure 1.
Flanked and unflanked acuity are equally easy to measure. However, flanked acuity is much preferable for screening, as shown in Figure 12. An optimal acuity cut off (Figure 12a) will pass well-refracted normals and detect patients with low acuity, but it cannot detect patients with normal acuity, which here include one strabismic amblyope and most of the apperceptive agnosics. Better, the flanked acuity tests (Figure 12b, c) pass only observers who have both the requisite acuity and critical spacing. With a criterion of 0.18°, both flanked acuity tests pass all the normals. The loose chart (b) detects all but one of the patients. The tight chart detects all the patients.
There is a substantial clinical literature on measuring acuity, with and without flankers, to screen for amblyopia. Our findings are mostly consistent with that literature, with one important caveat. That literature treats all flanker effects alike. No attempt is made to distinguish crowding (distinguished by critical spacing independent of target size) from overlap masking (distinguished by critical spacing proportional to target size). The clinical literature has instead assumed that the flanker effect in strabismic amblyopia (i.e., crowding) is simply an exaggerated version of the flanking effect in the central vision of normals and purely anisometropic amblyopes (i.e., overlap masking). Fifty years ago, Thomas-Decortis (1959) found the same modest flanker effect in normals and purely anisometropic amblyopes and a much bigger flanker effect in strabismic amblyopes. However, while confirming her results, Stuart and Burian (1962) confused the issue by advancing the unhelpful thesis “that all sensory phenomena observed in strabismic patients are exaggerations of pre-existing physiologic phenomena (p. 471).” They concluded “that crowding is a universal phenomenon, … correlated with … visual acuity … exaggerated with any form of strabismic amblyopia (p. 476).” The mistaken assumption that flanker effect has the same cause in strabismic amblyopia as in normal central vision persists to this day. This has led to two errors in the design of tests to screen for amblyopia. We commend three commercially available flanked acuity charts for having only moderately loose letter spacing (s = 1.5) and letter-like flankers: the Cambridge Crowding Cards, the Davidson-Eskridge illiterate E chart, and the Glasgow Acuity Cards (sold by Keeler as the LogMAR Crowding Test). To our knowledge, all other commercially available flanked acuity tests for amblyopia suffer from one or the other of two faults: Either the flankers are insufficiently close to the target or the flankers are insufficiently similar to the target.
Flankers are much more effective in crowding if they are similar to the target (Kooi, Toet, Tripathy, & Levi, 1994). With the same gap between target and flankers, flanking bars are much less effective than letters in crowding a target letter (Pelli, Song, & Levi, 2011).
Note that flankers identical to the target do not crowd target identification. (We have long known this, but this seems to be the first time it has been reported.) To crowd, flankers should not be the same letter as the target.
Our results suggest that makers of flanked acuity tests can improve sensitivity in detecting strabismic amblyopia by using target-like flankers very near to the target.
Optimizing the flanked acuity test
Most of the results reported here were collected with tight spacing, a letter-spacing factor s of 1.1 (Figure 1). What value of s would be optimal for a clinical test? For diagnostic sensitivity to crowding, one wants as big a difference as possible between thresholds with and without crowding. We recommend measuring flanked acuity with an optimally tight chart. Since the threshold spacing of crowding is independent of the chart's spacing factor, the chart's sensitivity to crowding is maximized by choosing the spacing factor s to minimize the threshold spacing in the absence of crowding. The optimum is in the tight range 1.1 < s < 1.4, and our model of overlap masking suggests an optimal spacing of s = 1.13. Using a loose chart reduces the measured difference between normal and strabismic amblyopes, making it harder to distinguish them.
Measuring acuity with a full letter chart or a line of letters is often considered more sensitive for detecting amblyopia than isolated letter acuity (Morad, Werker, & Nemet, 1999; Tommila, 1972; Wick & Schor, 1984). However, currently, the most common commercially available acuity charts have loose spacing, s > 1.4, making them less than optimal for measuring flanked acuity. For example, the Bailey-Lovie chart has s = 2, as does the widely used EDTRS chart. Three charts have tighter, but still loose, spacing (s = 1.5): Davidson-Eskridge illiterate E chart, the Cambridge Crowding Cards (Atkinson et al., 1986), and the Glasgow Acuity Cards (McGraw & Winn, 1993). As noted above, for best diagnostic sensitivity to crowding, flanked acuity should be measured with an optimally tight chart. The spacing factor s should minimize the threshold spacing in the absence of crowding.
We suggest that an identification test to screen for amblyopia should use a simple target, like a letter, with optimally spaced target-like flankers. Such flankers will expose abnormal crowding and thus detect strabismic amblyopes. The probability of guessing should be kept down by randomly selecting each target letter to be displayed from at least five possible letters (Pelli & Bex, 2013).
Stereoacuity
Ciner et al. (2014) report encouraging results for preschooler screening by random dot stereoacuity. We find that stereoacuity is almost perfectly correlated with crowding; in our subjects, screening for stereoacuity detects strabismic amblyopes about as well as flanked acuity does. However, stereoacuity might not be as good as acuity (flanked or unflanked) in detecting the pure acuity deficit of anisometropic amblyopes and pure refractive error.
Extending our result to children
Little is known about the developmental time course of crowding (Kwon et al., 2007; Pelli & Tillman, 2008). Many share Atkinson and Braddick's (1983) impression that “By age 3 years resolution acuity is very close to adult performance, but at 5 years ‘crowding' effects may still impair performance on practical acuity tasks more than for the adult” (Atkinson, 1991; Jeon, Hamid, Maurer, & Lewis, 2009, see Manny, Fern & Loshin, 1987 for a contrary view). Finding that acuity matures before crowding does would be further evidence of dissociation of crowding and acuity. Determining the best criterion value for screening should be based on testing of children.
7b. Demonstrations of our screening recommendations
Our results show that the foveal vision of strabismic amblyopes is well-modeled by the crowding of normal peripheral vision. That finding on adults indicates that two improvements to existing charts would greatly increase their diagnostic sensitivity to strabismic amblyopia, with no loss of specificity, at least in adults. First, the flankers should be letter like, not bars (Figure 13). Second, the flankers should be closer to the target letter, i.e., more tightly spaced (Figure 14).
Figure 13.
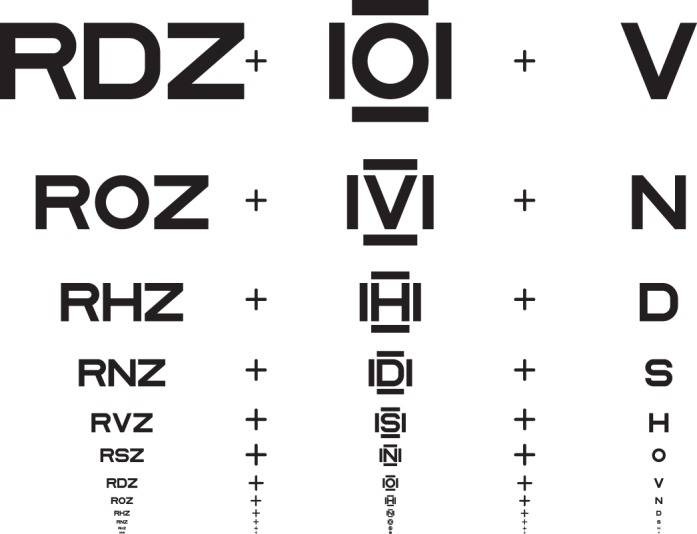
Flankers should be target like. The clinical literature on screening for amblyopia has often implicitly assumed that “contour interaction” (overlap masking) and crowding are the same thing, but they are not, as this figure demonstrates. In the normal fovea, where flanked acuity is limited by overlap masking, surrounding the target with nearly contiguous bars (“contours”) or letters has the same effect, which you may witness by comparing these three eye charts. (In the first column, use only the middle letter for testing; the outer letters are not fully flanked.) In any row, across the three charts, all three targets have the same size, which drops by a factor of
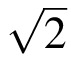 from row to row. The left column has letter flankers (R, Z), the middle column has bar flankers, and the right column is unflanked. The conclusions of this demo depend only on comparing charts, side by side, at any viewing distance. If you like, viewing from at least 2 m will eliminate any concern that you might be limited by the resolution of this page. As a normal observer, looking directly at each target, you will find that both kinds of flanker are equally effective. Letters and bars (left and middle columns) raise threshold one row above that for unflanked acuity (right column). For a given gap between target and flanker, you have the same flanked acuity (threshold row) with letter and bar flankers. This is overlap masking. Simulating a strabismic amblyope, please fix your gaze on a + sign and peripherally view the target (to right or left). As a (simulated) strabismic amblyope, you are limited by crowding. Unlike overlap masking, crowding is very sensitive to the degree of similarity of target and flanker. The letter flankers are much more effective than the bars, because they are more similar to the target, even though, having the same gap, they are farther away, center to center. Thus, in existing tests, replacing bar flankers by more target-like flankers will worsen the flanked acuity of the strabismic amblyopes without affecting the flanked acuity of normals. This will increase the separation of the two populations, increasing the power of the test to detect strabismic amblyopes among normals.
from row to row. The left column has letter flankers (R, Z), the middle column has bar flankers, and the right column is unflanked. The conclusions of this demo depend only on comparing charts, side by side, at any viewing distance. If you like, viewing from at least 2 m will eliminate any concern that you might be limited by the resolution of this page. As a normal observer, looking directly at each target, you will find that both kinds of flanker are equally effective. Letters and bars (left and middle columns) raise threshold one row above that for unflanked acuity (right column). For a given gap between target and flanker, you have the same flanked acuity (threshold row) with letter and bar flankers. This is overlap masking. Simulating a strabismic amblyope, please fix your gaze on a + sign and peripherally view the target (to right or left). As a (simulated) strabismic amblyope, you are limited by crowding. Unlike overlap masking, crowding is very sensitive to the degree of similarity of target and flanker. The letter flankers are much more effective than the bars, because they are more similar to the target, even though, having the same gap, they are farther away, center to center. Thus, in existing tests, replacing bar flankers by more target-like flankers will worsen the flanked acuity of the strabismic amblyopes without affecting the flanked acuity of normals. This will increase the separation of the two populations, increasing the power of the test to detect strabismic amblyopes among normals.
Figure 14.
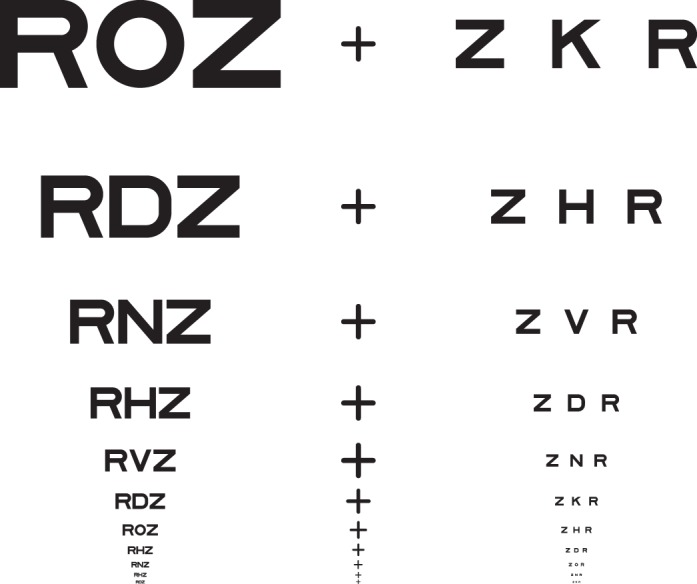
Spacing should be tight. Two charts with different spacing factors (i.e., spacing/size): a tight 1.1× (which we recommend) and a loose 2× (which is typical of the commercially available tests). Each line of each chart displays a target letter between two flankers (R,Z). Acuity imposes a floor on the spacing threshold: An observer with acuity A reading a chart with spacing factor s cannot read any letters with spacing below sA. The 2A floor of a 2× chart hardly increases the spacing at flanked acuity threshold of a strabismic amblyope (for whom Scrowding > 1.84A), but greatly increases that of a normal observer (for whom Smasking ≈ 1.4A if s = 1.1, and may be even lower with slightly larger s). A few commercially available letter-flanked acuity tests have a spacing factor of 1.5×, and the rest have 2×. Assuming you have normal vision, you can test this spacing-factor effect on yourself, first foveally—as a normal—and then peripherally—modeling a strabismic amblyope. Each column has a different spacing factor: 1.1× on the left and 2× on the right. The columns are aligned so that both targets in each row have the same center-to-center spacing S of target to flanker. The whole three-letter triplet shrinks by a factor of
 from row to row. As a normal observer, look directly at each target, the middle letter of each triplet. Notice that, viewing directly, you read one more row (smaller spacing) with the tighter spacing (left column). On the left, you are limited by overlap masking by the flankers (threshold spacing 1.4A), well above the spacing floor of 1.1A imposed by the chart, given your acuity A. On the right, the flankers have no effect and the spacing at your flanked acuity threshold is at the 2A floor imposed by acuity with s = 2. Thus threshold with the loose chart is 2/1.4 higher, which is roughly
from row to row. As a normal observer, look directly at each target, the middle letter of each triplet. Notice that, viewing directly, you read one more row (smaller spacing) with the tighter spacing (left column). On the left, you are limited by overlap masking by the flankers (threshold spacing 1.4A), well above the spacing floor of 1.1A imposed by the chart, given your acuity A. On the right, the flankers have no effect and the spacing at your flanked acuity threshold is at the 2A floor imposed by acuity with s = 2. Thus threshold with the loose chart is 2/1.4 higher, which is roughly
 , one line on these charts. Simulating a strabismic amblyope, fixate the central + sign in the top row. While still fixating, try to identify the target to the left and the target to the right. If you succeed, then proceed to the next row down, until you fail. Notice that, as a strabismic amblyope, limited by crowding, you have the same spacing threshold (row) with both charts (left and right). Thus, tightening the spacing (from 2× to 1.1×) reduced normal threshold spacing (above) but does not affect threshold of the strabismic amblyope. This increases the separation of the two populations, increasing the power of the test to detect strabismic amblyopes among normals. If you like, viewing from at least 2 m will eliminate any concern that you might be limited by the resolution of this page. In fact, the point demonstrated here is independent of the source of the acuity-limiting blur. No matter whether the limiting blur arises in the chart, the retinal image, or the neural representation, the more tightly spaced chart is better at detecting strabismic amblyopia.
, one line on these charts. Simulating a strabismic amblyope, fixate the central + sign in the top row. While still fixating, try to identify the target to the left and the target to the right. If you succeed, then proceed to the next row down, until you fail. Notice that, as a strabismic amblyope, limited by crowding, you have the same spacing threshold (row) with both charts (left and right). Thus, tightening the spacing (from 2× to 1.1×) reduced normal threshold spacing (above) but does not affect threshold of the strabismic amblyope. This increases the separation of the two populations, increasing the power of the test to detect strabismic amblyopes among normals. If you like, viewing from at least 2 m will eliminate any concern that you might be limited by the resolution of this page. In fact, the point demonstrated here is independent of the source of the acuity-limiting blur. No matter whether the limiting blur arises in the chart, the retinal image, or the neural representation, the more tightly spaced chart is better at detecting strabismic amblyopia.
Summary and conclusions
1a. Size and spacing
We systematically explore the size and spacing requirements for identifying a letter among other letters by normal and amblyopic observers and present a very simple model: Our results trace out the visual size and spacing limits imposed by acuity, overlap masking, and crowding (Equations 9 and 18). In general, for each eccentricity, we find that a flanked letter is legible if and only if it respects all three limits: acuity, crowding, and overlap masking.
1b. Crowding and overlap masking
Object recognition can be impaired by flankers. This can be due to either crowding or overlap masking. Flankers similar to the target can produce crowding or overlap masking of the target. However, crowding is much more selective. Crowding is abolished by replacing the flankers by letters that are identical to the target or objects (bars) that are very different from the target. Overlap masking endures such replacement. Furthermore, we find that the critical spacing of crowding is independent of acuity (5, below), whereas overlap masking is tied to acuity (Equations 3 and 5).
2. Linear dependence on eccentricity
For normals and amblyopes it is commonly found that acuity and critical spacing of crowding depend linearly on eccentricity, A = a(φ + φA) and Scrowding = b(φ + φcrowding), and that they approach normal values at large eccentricity, which implies that a and b are conserved. Thus, any individual patient's ability to identify letters, at all sizes, spacings, and eccentricities, is specified by the two add-on eccentricities φA and φcrowding, which can be estimated by measuring just the flanked and unflanked acuity.
3. Eccentricity and blur are good models of amblyopia
Amblyopia is a developmental neural deficit. We find that the adult impairment retains the character of the childhood condition that caused it. Eccentric viewing during childhood due to strabismus results in amblyopia that is much like eccentric viewing. Optical blur of one eye during childhood due to anisometropia without strabismus results in amblyopia that is much like blur. Strabismic amblyopia is mainly spacing limited, like normal vision at greater eccentricity, and non-strabismic (purely anisometropic) amblyopia is mainly size limited, like normal central vision with added blur.
The threshold spacing of strabismic amblyopes is limited by crowding, whereas that of purely anisometropic amblyopes is limited by overlap masking. Equivalent eccentricity and blur accurately model effects of size and spacing in strabismic and purely anisometropic amblyopia, respectively. Additional tests confirm that both kinds of amblyopia are in fact neural deficits of perception, not just poor optics or fixation (Appendix C).
4a. The spacing:acuity ratio S/A
We find that flanked and unflanked acuity, together, as the spacing:acuity ratio, distinguish strabismic from purely anisometropic amblyopia, in nearly perfect agreement with lack of stereopsis. Alone, neither flanked nor unflanked acuity can distinguish the two kinds of amblyopia. Among our 18 amblyopes, we find near-perfect agreement of three diagnostic indicators: history of strabismus, absence of stereopsis, and a high spacing:acuity ratio (S/A > 1.84). The 1.84 criterion is based on our adult results with medium-contrast brief white letters. The optimal criterion for children and the more common high-contrast black-letter chart may be different and should be determined for the particular age group and kind of chart.
4b. Clinical assessment of crowding
To distinguish strabismic from purely anisometric amblyopia, our study of adults recommends measuring flanked and unflanked acuity, computing the spacing: acuity ratio, and applying a criterion of 1.84. A developmental study is needed to establish the appropriate criterion value for children. To optimize detection of crowding, the flanked acuity chart should be optimally tight. To maximize sensitivity to crowding—i.e., detect the difference between patients and normals—the chart should be optimally tight (i.e., minimize S in Equation 11). This optimum is in the range 1.1 < s < 1.4, and may be near s = 1.13.
5. Double dissociation
It has often been assumed that critical spacing is tied to acuity, as in overlap masking, but, in concert with Strappini et al. (submitted), we report a double dissociation of acuity and crowding: Different clinical conditions selectively impair one or the other, showing that acuity and crowding are functionally distinct and separately modifiable. Models of human object recognition will need to accommodate this newly discovered independence of acuity and crowding.
Historically, it has been confusing that letter identification, and object recognition in general, are subject to two different kinds of spacing limitation. Overlap masking is tied to acuity, Smasking = 1.4A (Equations 3 and 5), whereas crowding is independent of acuity. Both matter in amblyopia. Strabismic amblyopes have increased crowding, but purely anisometropic amblyopes do not. For diagnosis or basic characterization, once acuity has been measured, the overlap masking is practically known, but the extent of crowding is an important independent dimension that may be worth measuring.
6. Three paths to crowding
Crowding is negligible in the normal fovea, but three very different histories—normal peripheral development, childhood strabismus, and accidental brain lesions in adulthood—can all result in severe crowding. It is surprising that each patient's crowding in central vision is always well-modeled by the normal periphery at some particular eccentricity. Whatever the etiology, human visual object recognition arrives at a similar computational structure whose crowding is well characterized by one number, the equivalent eccentricity, which may gauge available neural resources.
7a. Diagnosis
For clinical use, plotting each patient as a point, threshold size versus acuity, for several diagnostic groups, reveals the diagnostic power of flanked acuity.
7b. Clinical screening
To quickly screen the general population of children to identify those who may have amblyopia or might otherwise benefit from professional eye care, we endorse the current practice of measuring flanked acuity. However, with the possible exception of the Cambridge Crowding Cards, the Davidson-Eskridge chart, and the Glasgow Acuity Cards (aka Keeler LogMAR Crowded Test), our results suggest that the sensitivity of the available flanked acuity tests is curtailed by use of flankers that are insufficiently similar and near to the target letter to produce strong crowding. Our model predicts that these tests can be improved by using tightly spaced target-like flankers (as demonstrated in Figures 13 and 14). This will increase sensitivity for detecting strabismic amblyopes among normals, while maintaining specificity. In our sample, all three well-corrected normals could identify letters with 0.2° spacing, and none of the 18 amblyopes could.
Marginal letters, flanked on only one side, like the R and Z in Figures 13 and 14, may escape crowding (Bouma, 1973) and should be ignored in scoring. Making the flankers identical to the target hardly affects overlap masking but abolishes crowding of target identity (Appendix B). To crowd the target's identity, the flankers must not be identical to the target.
When screening, if you can only measure one thing, measure flanked acuity with optimally spaced target-like flankers, and calculate the threshold spacing, as it is a fundamental dimension of vision and is robust for contrast, duration, and luminance. If you have the luxury of measuring a second thing, add acuity. You can then calculate the spacing:acuity ratio and the add-on eccentricities, φA and φcrowding, which are enough to predict your observer's acuity and threshold spacing at any eccentricity.
Acknowledgments
These experiments grew out of discussions between Dennis Levi and Denis Pelli on how to characterize amblyopia. Shuang Song made all the measurements and wrote the first draft (5,000 words), as a PhD student in Dennis Levi's lab under his supervision. This is Draft 112, now 22,000 words. Over the course of these many drafts, Denis Pelli suggested several new measurements (including effects of blur and pinhole), rewrote most of the paper, and added the size-and-spacing model of legibility (Equations 1–18), the recommendations and demos for improved screening of amblyopia (Figures 13, 14, and 16), and the double-dissociation finding. The order of authors' names in our byline follows the tradition of making the student first author when the paper is based on her thesis work and designates as “senior” (last) author the person who contributed the most (APA, 2002). Earlier versions of Figures 13 and 14 were presented at the ABC Atkinson-Braddick Celebration, Oxford University, 27–28 September, 2010, and at VSS in 2011. Thanks to an anonymous reviewer for suggesting the eccentricity scaling analysis presented in Figure 3. Thanks to Susana Chung, Jian Ding, David Hoffman, Deborah Levy (abstract and conclusions), Roger Li, Najib Majaj (Figure 16), Sarah Rosen, Toni Saarela, Carol Seaholm Volow, Elizabeth Segal, Katharine Tillman, and Lauren Vale for helpful comments, and to Diana Balmori, Margaret Morton, and Cesar Pelli for help with earlier versions of the title. As a graduate student, Shuang Song was supported by a fellowship from UC Berkeley. Support was provided by US National Institutes of Health grants EY04432 to Denis Pelli and EY01728 to Dennis Levi.
Commercial relationships: none.
Corresponding author: Denis G. Pelli.
Email: denis.pelli@nyu.edu.
Address: Psychology & Neural Science, New York University, New York, NY, USA.
Appendix A
The generality of our results
In the current study, we asked observers to identify a briefly presented (200 ms) medium contrast (0.6) bright letter on a background of 30 cd/m2 with natural pupil size. These parameters differ from typical acuity testing in the clinic, where the letters are static, not flashed, are dark, not bright, and have high contrast. For example, Atkinson et al. (1988) measured visual acuity using a printed chart with static high-contrast (−0.9) black letters on a bright background (30 cd/m2). Similar conditions are encountered in most clinical settings for vision testing. When other conditions (e.g., letter font, center-to-center letter spacing, threshold criteria) are equal, “print-like letters” (i.e., computer-generated high-contrast black letters with unlimited viewing duration) and “CRT letters” (the conditions used in our main experiment) might yield different visual acuities and A'/A ratios. This raises the question of whether our findings generalize to the clinical situation. In order to address the question of generality and establish the connection between our experimental data and the visual acuities obtained with more commonly used print-like letter stimuli, we explored the effects of polarity, contrast, duration, pupil size, and background luminance on flanked and unflanked acuity. In this Appendix, Figure 15 and Table 3 summarize the main effects of these stimulus parameters as they relate to the generality of our results and our recommendations for the design of a screening test.
Figure 15.
Threshold spacing versus acuity for normal observers (see Figure 10 for amblyopes). (a) All conditions. (b) Expanded view of lower left. Contrast is denoted by gray level, lighter for lower contrast. Results for white/black letters are represented by open/filled symbols. Duration is denoted by size; “∞” in the legend indicates static presentation. Spacing is 1.1× (empty and filled circles, +) or 1.5× (squares, triangles, C, N). We assess the effect of image quality at fixation by comparing results with a 2 mm pinhole (triangle) or optical or digital blur (hourglass symbol). Dashed lines represent the crowding limits at 0° and 5° eccentricity. The dotted line represents the overlap masking limit with m = 1.4 (Equation 3). Except for the Cambridge Crowding Cards (C), and the “N-Z” test (N or Z), the rest of the data are for computerized testing on a CRT. The N represents the results for identifying one of four symbols (N, reflected N, Z, reflected Z) with or without four flankers on a printed card that that is otherwise like the Cambridge Crowding Cards (s = 1.5). The Z is similar, but for a tightly spaced chart (s = 1.1). Foveal data points from the literature are plotted as yellow symbols. Danilova and Bondarko (2007, yellow X) measured foveal acuities and critical spacings for several subjects under multiple conditions: Landolt C with flankers such as bars and gratings of different spatial frequencies, Tumbling E's, grating flanked by similar gratings of the same or higher spatial frequencies with fixed or random orientations. These conditions are not distinguished in the graph. Error bars show the standard errors across subjects. Note that the thresholds were not measured at a fixed criterion (e.g., 75% correct). Latham and Whitaker (1996, yellow square 1.5× and diamond 1.25×) measured foveal acuities and the critical spacings of the grating flanked by similar gratings at random orientations. The spacing factor s is fixed at either 1.5× (square) or 1.25× (diamond). The target and flankers scale proportionally. Their experimental condition is the most similar to ours. Since there are only two subjects, their data points were plotted individually instead of being averaged. Toet and Levi (1992, yellow circle) measured foveal acuities and the critical spacings of the tumbling letter T with similar flankers. Error bars show the standard errors across subjects.
Despite the wide range of conditions (polarity, contrast, duration), all the foveal data are clustered around the overlap-masking line (S = 1.4A). The peripheral results at 5° are all near the 5° crowding line.
Most of our data are for moderate to high contrast and 200 ms. We have explored a wide range of durations (13 ms to unlimited), contrast (0.15 to 0.9), and both contrast polarities. Peripheral (5° ecc.) flanked acuity (i.e., crowded) is relatively insensitive to contrast and duration. The log-log slope of flanked and unflanked acuity versus duration is −0.2. At 5° ecc., the log-log slope of flanked (or unflanked) acuity versus contrast is −0.13 (or −0.37).
Duration has the same weak effect on flanked and unflanked acuity in the fovea and periphery. It is well known that foveal acuity depends on contrast with a log-log slope of −0.5, consistent with our results (Table 3). The most striking fact in Table 3 is the near absence of an effect of contrast under conditions of crowding (5° ecc.). This is consistent with past results with gratings (Levi & Carney, 2009, 2011).
As we noted above, Figure 15a plots the spacing: acuity ratio S/A against unflanked acuity A for all the conditions that we tested, spanning a wide range of contrast, duration, polarity, pupil size (with and without 2 mm pinhole), and eccentricity (0° and 5°), for normal observers and amblyopes. In Figure 10, the horizontal line at S/A = 1.84 (dot dashed) is the criterion obtained from the current study, which distinguishes strabismic amblyopia (red) from anisometropic amblyopia (green). All the normal peripheral points (circles) are above the line, and all the foveal points (black), including blur (+ and ×) are below it.
Examining the normal foveal data more closely in Figure 15d, note that the spacing:acuity ratios are higher for 1.5× spacing letters (squares) than 1.1× spacing letters (circles). Looser spacing pushes the normal up higher (Figure 15d), closer to where strabismic amblyopes lie (Figure 15a). This effect of spacing on flanked threshold size is consistent with what was reported by Latham and Whitaker (1996), and it implies that tests with tight spacing are better at separating normal and strabismic amblyopic results. In the fovea, all 1.1× spacing data points are near the overlap masking line (dotted line), well below the criterion value of S/A = 1.84, but a few 1.5× spacing points (including measures using the Cambridge Crowding Cards) as well as data from several other studies (yellow symbols) are well above the S/A = 1.4 masking line and even exceed the 1.84 criterion. We will discuss these points below (Appendix B). We noted above that tightly spaced tests (e.g., 1.1×) are better at differentiating strabismic from anisometropic amblyopia, and Figure 15 indicates that this will generalize quite well to other testing conditions. Furthermore, a tight spacing is also better at differentiating strabismic amblyopia from normal foveal vision.
This finding suggests that a vision test with tight spacing is more effective in screening for strabismic amblyopia. Thus, measuring acuity and flanked acuity with a tight spacing provides a screening test for strabismic amblyopia that is robust across stimulus conditions.
Appendix B
Crowding in the fovea
We wondered whether the threshold spacing measured in the normal fovea is due to crowding or overlap masking. We have already presented rigorous tests for each hypothesis, and, surprisingly, both hypotheses survive our testing with brief medium-contrast. The threshold spacing in the normal fovea could be either crowding or overlap masking.
On the one hand, Figure 3 supports crowding, showing that performance is invariant when the image size scales with padded eccentricity, φ + φcrowding, for all eccentricities tested, including zero. Scaling with the padded eccentricity φ + φcrowding, is a hallmark of crowding.
On the other hand, Figures 2 and 3 show that the spacing:acuity ratio S/A at fixation for our normal observers was about 1.4, which, when s = 1.1, is the hallmark of overlap masking (as shown in Figure 4).
Table 3.
Effects of contrast and duration on acuity (A) and flanked acuity (A'). Each log-log slope of A (or A') versus contrast is averaged over the various durations, and each log-log slope of A (or A') versus duration is averaged over the various contrasts. Greater absolute value of the log-log slope indicates a stronger effect. The spacing factor s is 1.1.
|
Ecc. (deg) |
Duration (ms) |
Contrast |
Slope of log A vs. log contrast |
Slope of log A' vs. log contrast |
Slope of log A vs. log duration |
Slope of log A' vs. log duration |
| 5 | 100, 200, 400 | 0.15 to 0.9 | −0.37 | −0.13 | −0.19 | −0.21 |
| 0 | 100, 200, 400 | 0.15 to 0.9 | −0.43 | −0.26 | −0.22 | −0.21 |
| 0 | 13, 26, 53 | 0.3, 0.6 | −0.58 | −0.58 | −0.54 | −0.54 |
Figure 16.
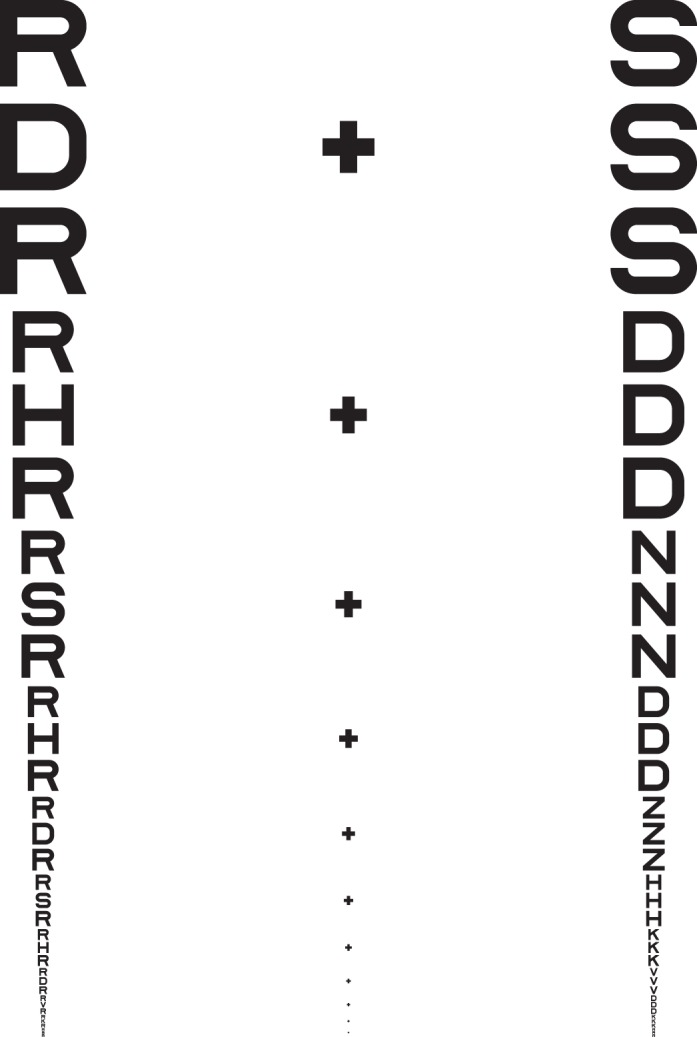
Distinguishing crowding from overlap masking. When a target letter is among flanking letters, the flankers can impair target recognition in two ways: crowding and overlap masking. These effects have different explanations and phenomenology but have not always been easy to distinguish. Here we introduce a new diagnostic test. Making the flankers identical to the target abolishes crowding yet hardly affects overlap masking. Regan, Giaschi, Kraft, and Kothe (1992) introduced a repeated-letter chart to tolerate errors in fixation or selection. Here we exploit its immunity to crowding. The threshold spacing of peripheral vision is normally due to crowding. (The conclusions of this demo depend only on comparing the two charts, side by side, at any viewing distance. If you like, viewing from at least 2 m will eliminate any concern that you might be limited by the resolution of this page.) Please fixate the top plus sign and try to identify the middle letter in the vertical triplets to the left and right. Ignore the two flankers above and below each target letter. On the left, the flankers (R) are different from the target. On the right, they are identical to the target. You will find that you can read more lines in the right column than in the left column. Your left-column threshold is limited by the severe crowding of peripheral vision; your threshold spacing is several times larger than your acuity A. Your right-column threshold is limited by overlap masking, a spacing of 1.4A, which is less than twice your acuity, allowing you to read more lines. In central vision, we saw above that crowding and overlap masking are both viable explanations for the measured threshold spacing of 1.4A. Using central vision to directly fixate each target we find that we can read down to the same level on both sides of the chart. There is no effect of making the flankers identical to the target. Since abolishing crowding has no effect, any crowding present must have a threshold spacing less than or equal to that of the overlap masking. Note that most charts probe critical spacing radially from the point of fixation; the target and flankers all lie on the same radial line. This chart probes critical spacing tangentially; the target and flankers lie on a line that is orthogonal to the radial line connecting target to fixation. Critical spacing tangentially is about half the critical spacing radially (Toet & Levi, 1992).
Some previous studies did not find crowding in the fovea, possibly because they used stimuli that are either too big or too faint contrast for crowding to occur (Levi, Klein, & Hariharan, 2002; Pelli et al., 2004; Strasburger, Harvey, & Rentschler, 1991). See Levi (2008) and Levi and Carney (2011) for a discussion of foveal crowding. Nevertheless, earlier experiments that used simple stimuli (e.g., line segments, “T”) did show crowding-like phenomena with tiny stimuli in the fovea, and our results confirm those findings (Latham & Whitaker, 1996; Levi, Klein, & Aitsebaomo, 1985; Toet & Levi, 1992).
To understand why neither hypothesis was rejected, note that the normal foveal results with brief letters (gray and black in Figure 15d) cluster near the intersection of the crowding and overlap masking hypothesis lines intersect. To test this further, we developed a new static high-contrast N-Z chart with 1.1× spacing (Figure 15, symbol Z), in which the target (and flankers) is N or Z or a mirror-reversed version of N or Z. We also created a digitally blurred version (Figure 15, symbol Z). For normal observers viewing foveally (unlimited duration), we found a threshold size A = 0.048 ± 0.002°, and threshold spacing, S = 0.116 ± 0.012°. Digital blur increased A to 0.068 ± 0.003°, but importantly had no effect on S (Figure 15, symbol Z). Thus, using high-contrast targets with unlimited exposure and simple features, we get down to smaller threshold size, finding a spacing threshold in the fovea that is too large for overlap masking and is independent of size and blur, and thus is diagnostic of crowding.
Our finding of a very small spacing limit in the normal fovea may appear to be at odds with a recent report by Siderov, Waugh, and Bedell (2012). They measured letter identification performance (percent correct) for near acuity foveal letters of different contrast levels, and therefore different sizes, as a function of the spacing of flanking bars. They report that flanking bars reduced performance even for their largest (10 arc min), low contrast letters. However, for all three letter sizes, performance declined when the edge-to-edge distance of the flankers was less than roughly 2 arc min, and target and flankers were both optically (due to the eye's blur function) and neutrally overlapping. We suggest that their “contour interaction” may reflect both overlap masking and crowding (Coates & Levi, 2014).
Figure 16 applied yet another test to try to distinguish whether foveal threshold spacing is due to crowding or overlap masking. It shows that eliminating crowding has no effect, showing that any crowding present must have a threshold spacing less than or equal to that of the overlap masking.
Appendix C
Null hypotheses
Pinhole relieves optical blur but not amblyopia
Our results show that the performance of purely anisometropic amblyopes is well modeled by adding optical blur to normal central vision. However, the results we have already presented do not exclude the null hypotheses possibility that the purely anisometropic amblyopes are indeed limited by optical blur, perhaps because of higher order optical aberrations or because their accommodation is less accurate. We doubt these optical accounts for two reasons. First, a recent study has shown that there is no significant difference in higher order aberrations between children with amblyopia of any kind and children without amblyopia (Kirwan & O'Keefe, 2008). Second, an optical explanation would predict that the purely anisometropic amblyope's pinhole acuity would be better than his acuity with normal pupil size (about 3–4 mm), since the pinhole greatly decreases the effect of defocus and eliminates most other optical aberrations (Artal, Marcos, Iglesias, & Green, 1996; Campbell & Gubisch, 1966).
Our measurements (Figure 17) confirm the optical benefit of the pinhole in normal vision and reject the null hypothesis that the purely anisometropic-amblyopic deficit is optical. We applied various amounts of defocus to blur the normal, viewing directly. We found that at a similar acuity level, the blurred normal benefitted greatly from the pinhole (1.5 mm), but the purely anisometropic amblyope did not. However, in addition to the pinhole's visually beneficial effect of narrowing the eye's point spread function, it also has the deleterious effect of reducing the retinal illuminance by roughly fivefold. We used a neutral density filter (density 0.7, 20% transmission) to assess the isolated effect of the change in retinal illuminance on the normal observer. For a normal observer with +1.5 D defocus (which is the equivalent blur for SW) the pinhole improves acuity by 2.5×, but a similar luminance reduction produced by the neutral density filter worsens acuity by 1.1×. The large effect of the pinhole and the small effect of pure luminance reduction (by the neutral density filter) together suggest that luminance reduction plays a negligible role in the effect of the pinhole on acuity.
Figure 17.
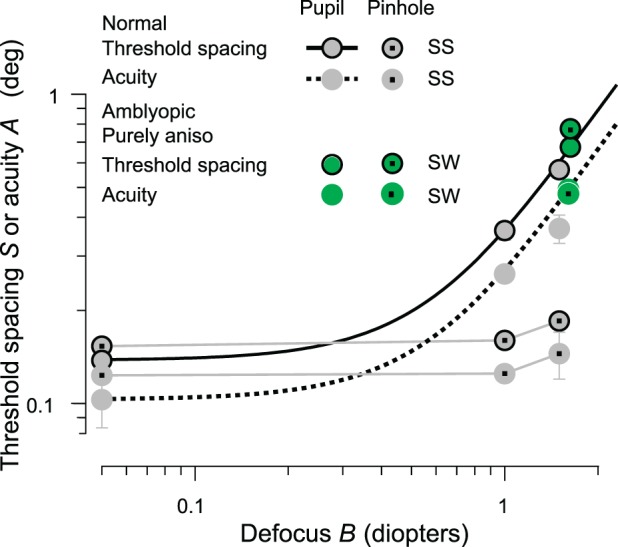
Assessing the blur model of purely anisometropic amblyopia by measuring effects of blur. As a function of defocus, for normal (gray) and purely anisometropic-amblyopic (green) central vision, symbols with and without black edges represent threshold spacing and acuity, respectively. Symbols with a central dot indicate that a pinhole was used. The natural pupil diameter is 3–4 mm and the pinhole diameter is 1.5 mm. The dotted curve is our equivalent-blur model for purely anisometropic amblyopia. Normal data are plotted against defocus. Purely anisometropic-amblyopic data are plotted against the corresponding equivalent blurs (to match acuity of the normal when both have natural pupils) for that observer. The spacing:acuity ratios S/A for the blurred normal (1.4 ± 0.03) and the amblyope (1.4) are not significantly different from m = 1.4, indicating that the threshold spacing is limited by overlap masking.
Thus, since the equivalent blur deficit, unlike real optical blur, is not relieved by a pinhole, we reject the null hypothesis that the purely anisometropic-amblyopic equivalent-blur deficit is due to optical blur. The amblyopic impairment modeled by equivalent blur must be a neural deficit in perception.
Strabismic amblyopes fixate well enough
Our results show that strabismic amblyopic performance is well modeled by increased eccentricity. The null hypothesis would be simply that the strabismic amblyopes fixate poorly and that their visual acuities were therefore indeed measured in the periphery. We used a direct ophthalmoscope to assess fixation of each observer by estimating the distance of the fixation star from the fovea. All the strabismic amblyopes except VC and SF have near-central fixation (<0.5°). These deviations are much too small to explain their (large) equivalent eccentricities (1°–31°). One of the exceptions, VC, has large steady temporal eccentric fixation (about 8°) in her amblyopic (right) eye. However, the equivalent eccentricity for VC (based on her spacing: acuity ratio) is 31°, which is much greater than her eccentric fixation (8°). Therefore, her amblyopia cannot be accounted for by eccentric fixation. The other exception, SF, fixates unsteadily with his amblyopic eye, initially at fixation, and then sometimes at fixation but sometimes several degrees to the right of fixation. However, in our experiment, each stimulus is brief (200 ms), so unsteady fixation is unlikely to affect the measured visual acuity (Higgins, Daugman, & Mansfield, 1982). This may help to explain why SF's equivalent eccentricity (1°) is small compared to other strabismic amblyopes, in spite of the fact that he has unsteady fixation with drifts of up to several degrees away from the designated point for fixation. If SF were typically fixating extrafoveally, we would expect that he would have a larger, rather than a smaller, equivalent eccentricity. Thus we reject the null hypothesis for all of our strabismic observers. The amblyopic deficit modeled by equivalent eccentricity is not simply eccentric fixation and must be a neural deficit in foveal vision.
Contributor Information
Shuang Song, Email: songsh@berkeley.edu.
Dennis M. Levi, Email: dlevi@berkeley.edu.
Denis G. Pelli, Email: denis.pelli@nyu.edu.
References
- American Psychological Association. (2002). Ethical principles of psychologists and code of conduct. American Psychologist , 57 (12), 1060–1073, Sec. 8.12c. http://www.apa.org/ethics/code/index.aspx?item=11#812c [PubMed] [Google Scholar]
- Artal P., Marcos S., Iglesias I., Green D. G. (1996). Optical modulation transfer and contrast sensitivity with decentered small pupils in the human eye. Vision Res earch, 36 (22), 3575–3586 [DOI] [PubMed] [Google Scholar]
- Atkinson J. (1991). Review of human visual development: Crowding and dyslexia. London: MacMillan Press; [Google Scholar]
- Atkinson J., Anker S., Evans C., Hall R., Pimm-Smith E. (1988). Visual acuity testing of young children with the Cambridge Crowding Cards at 3 and 6 m. Acta Ophthalmologica (Copenhagen), 66 (5), 505–508 [DOI] [PubMed] [Google Scholar]
- Atkinson J., Braddick O. (1983). Assessment of visual acuity in infancy and early childhood. Acta Ophthalmologica, 61 (S157), 18–26, doi:10.1111/j.1755-3768.1983.tb03927.x [DOI] [PubMed] [Google Scholar]
- Atkinson J., Braddick O., Nardini M., Anker S. (2007). Infant hyperopia: Detection, distribution, changes and correlates-outcomes from the Cambridge infant screening programs. Optometry & Vision Science , 84 (2), 84–96 [DOI] [PubMed] [Google Scholar]
- Atkinson J., Pimm-Smith E., Evans C., Harding G., Braddick O. (1986). Visual crowding in young children. Documenta ophthalmologica. Proceedings series , 45, 201–213 [Google Scholar]
- Behrmann M., Kimchi R. (2003). What does visual agnosia tell us about perceptual organization and its relationship to object perception? Journal of Experimental Psychology: Human Perception and Performance , 29 (1), 19–42 [DOI] [PubMed] [Google Scholar]
- Behrmann M., Moscovitch M., Winocur G. (1994). Intact visual imagery and impaired visual perception in a patient with visual agnosia. Journal of Experimental Psychology: Human Perception and Performance, 20 (5), 1068 [DOI] [PubMed] [Google Scholar]
- Behrmann M., Williams P. (2007). Impairments in part-whole representations of objects in two cases of integrative visual agnosia. Cognitive Neuropsychology, 24 (7), 701–730 [DOI] [PubMed] [Google Scholar]
- Bonneh Y. S., Sagi D., Polat U. (2004). Local and non-local deficits in amblyopia: Acuity and spatial interactions. Vision Research , 44 (27), 3099–3110 [DOI] [PubMed] [Google Scholar]
- Boucart M., Moroni C., Despretz P., Pasquier F., Fabre-Thorpe M. (2010). Rapid categorization of faces and objects in a patient with impaired object recognition. Neurocase , 16 (2), 157–168 [DOI] [PubMed] [Google Scholar]
- Bouma H. (1970). Interaction effects in parafoveal letter recognition. Nature, 226 (5241), 177–178 [DOI] [PubMed] [Google Scholar]
- Bouma H. (1973). Visual interference in the parafoveal recognition of initial and final letters of words. Vision Research , 13, 767–782 [DOI] [PubMed] [Google Scholar]
- Brainard D. H. (1997). The Psychophysics Toolbox. Spatial Vision, 10 (4), 433–436 [PubMed] [Google Scholar]
- Buxbaum L. J., Glosser G., Coslett H. B. (1999). Impaired face and word recognition without object agnosia. Neuropsychologia , 37 (1), 41–50 [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Gubisch R. W. (1966). Optical quality of the human eye. The Journal of Physiology, 186 (3), 558–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. T., Levi D. M., Legge G. E. (2001). Spatial-frequency and contrast properties of crowding. Vision Research, 41 (14), 1833–1850 [DOI] [PubMed] [Google Scholar]
- Chung S. T. L. (2007). Learning to identify crowded letters: Does it improve reading speed? Vision Research , 47 (25), 3150–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. T. L., Li R. W., Levi D. M. (2012). Learning to identify near-acuity letters, either with or without flankers, results in improved letter size and spacing limits in adults with amblyopia. PLoS ONE, 7 (4), e35829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciner E. B., Ying G. S., Kulp M. T., Maguire M. G., Quinn G. E., Orel-Bixler D., Huang J. (2014). Stereoacuity of preschool children with and without vision disorders. Optometry and Vision Science , 91 (3), 351–358 doi:10.1097/OPX.0000000000000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda K. J., Levi D. M., Selenow A. (1991). Amblyopia: Basic and clinical aspects. Boston: Butterworth-Heinemann; [Google Scholar]
- Clarke M. P., Wright C. M., Hrisos S., Anderson J. D., Henderson J., Richardson S. R. (2003). Randomised controlled trial of treatment of unilateral visual impairment detected at preschool vision screening. BMJ: British Medical Journal, 327 (7426), 1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates D., Levi D. M. (2014). Contour interaction in foveal vision: A response to Siderov, Waugh & Bedell. Vision Research , 96, 140–144 doi:10.1016/j.visres.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutch S., Warrington E. (2007). Foveal crowding in posterior cortical atrophy: A specific early-visual-processing deficit affecting word reading. Cognitive Neuropsychology, 24 (8), 843–866 [DOI] [PubMed] [Google Scholar]
- Crutch S. J., Warrington E. K. (2009). The relationship between visual crowding and letter confusability: Towards an understanding of dyslexia in posterior cortical atrophy. Cognitive Neuropsychology, 26 (5), 471–498 [DOI] [PubMed] [Google Scholar]
- Danilova M. V., Bondarko V. M. (2007). Foveal contour interactions and crowding effects at the resolution limit of the visual system. Journal of Vision , 7 (2): 3 1–18, http://www.journalofvision.org/content/7/2/25, doi:10.1167/7.2.25. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Renzi E. (1996). Le agnosie visive. In Denes G., Pizzamiglio L. (Eds.), Manuale di Neuropsicologia Cognitiva. Normalità e Patologia dei Processi Cognitivi (p. 1426). Bologna, Italy: Zanichelli: [Google Scholar]
- Delvénne J. F., Seron X., Coyette F., Rossion B. (2004). Evidence for perceptual deficits in associative visual (prosop)agnosia: A single-case study. Neuropsychologia , 42 (5), 597–612 [DOI] [PubMed] [Google Scholar]
- Dutton G. N., Cleary M. (2003). Should we be screening for and treating amblyopia? BMJ: British Medical Journal , 327 (7426), 1242–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M. I., Reinecke R. D., Simons K. (1983). Preschool vision screening for amblyopia and strabismus. Programs, methods, guidelines, 1983. Survey of Ophthalmology, 28 (3), 145–163 [DOI] [PubMed] [Google Scholar]
- Ellemberg D., Hess R. F., Arsenault A. S. (2002). Lateral interactions in amblyopia. Vision Research, 42 (21), 2471–2478 [DOI] [PubMed] [Google Scholar]
- Elliott S. L., Georgeson M. A., Webster M. A. (2011). Response normalization and blur adaptation: Data and multi-scale model. Journal of Vision, 11 (2): July, 1–18, http://www.journalofvision.org/content/11/2/7, doi:10.1167/11.2.7. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D. B., Whitaker D., Bonette L. (1990). Differences in the legibility of letters at contrast threshold using the Pelli-Robson chart. Ophthalmic and Physiological Optics, 10 (4), 323–326 [DOI] [PubMed] [Google Scholar]
- Flom M. C., Weymouth F. W., Kahneman D. (1963). Visual resolution and contour interaction. Journal of the Optical Society of America A, 53 (9), 1026–1032 [DOI] [PubMed] [Google Scholar]
- Foulsham T., Barton J. J., Kingstone A., Dewhurst R., Underwood G. (2009). Fixation and saliency during search of natural scenes: The case of visual agnosia. Neuropsychologia , 47 (8-9), 1994–2003 [DOI] [PubMed] [Google Scholar]
- Funnell E., Wilding J. (2011). Development of a vocabulary of object shapes in a child with a very-early-acquired visual agnosia: A unique case. The Quarterly Journal of Experimental Psychology , 64 (2), 261–282 [DOI] [PubMed] [Google Scholar]
- Georgeson M. A., Sullivan G. D. (1975). Contrast constancy: deblurring in human vision by spatial frequency channels. The Journal of Physiology , 252 (3), 627–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilaie-Dotan S., Perry A., Bonneh Y., Malach R., Bentin S. (2009). Seeing with profoundly deactivated mid-level visual areas: Non-hierarchical functioning in the human visual cortex. Cerebral Cortex , 19 (7), 1687–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovagnoli A. R., Aresi A., Reati F., Riva A., Gobbo C., Bizzi A. (2009). The neuropsychological and neuroradiological correlates of slowly progressive visual agnosia. Neurological Sciences , 30 (2), 123–131 [DOI] [PubMed] [Google Scholar]
- Hariharan S., Levi D. M., Klein S. A. (2005). “Crowding” in normal and amblyopic vision assessed with Gaussian and Gabor C's. Vision Research, 45 (5), 617–633 [DOI] [PubMed] [Google Scholar]
- Hess R. F., Bradley A. (1980). Contrast perception above threshold is only minimally impaired in human amblyopia. Nature, 287 (5781), 463–464 [DOI] [PubMed] [Google Scholar]
- Hess R. F., Demanins R. (1998). Contour integration in anisometropic amblyopia. Vision Research, 38 (6), 889–894 [DOI] [PubMed] [Google Scholar]
- Hess R. F., Jacobs R. J. (1979). A preliminary report of acuity and contour interactions across the amblyope's visual field. Vision Research, 19 (12), 1403–1408 [DOI] [PubMed] [Google Scholar]
- Higgins K. E., Daugman J. G., Mansfield R. J. (1982). Amblyopic contrast sensitivity: Insensitivity to unsteady fixation. Investigative Ophthalmology & Visual Science , 23 (1), 113–120, http://www.iovs.org/content/23/1/113. [PubMed] [Article] [PubMed] [Google Scholar]
- Hiraoka K., Suzuki K., Hirayama K., Maori E. (2009). Visual agnosia for line drawings and silhouettes without apparent impairment of real-object recognition: a case report. Behavioural Neurology , 21 (3), 187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain Z., Webb B. S., Astle A. T., McGraw P. V. (2012). Perceptual learning reduces crowding in amblyopia and in the normal periphery. The Journal of Neuroscience, 32 (2), 474–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intriligator J., Cavanagh P. (2001). The spatial resolution of visual attention. Cognitive Psychology, 43 (3), 171–216 [DOI] [PubMed] [Google Scholar]
- Irvine R. S. (1945). Amblyopia ex anopsia. Observations on retinal inhibition, scotoma, projection, light difference discrimination and visual acuity. Transactions of the American Ophthalmological Society, 66, 527–575 [PMC free article] [PubMed] [Google Scholar]
- Jankowiak J., Kinsbourne M., Shalev R. S., Bachman D. L. (1992). Preserved visual imagery and categorization in a case of associative visual agnosia. Journal of Cognitive Neuroscience, 4 (2), 119–131 [DOI] [PubMed] [Google Scholar]
- Jeon S. T., Hamid J., Maurer D., Lewis T. (2009). The letter in the crowd: Developmental trajectory of single letter acuity and foveal crowding. Journal of Vision , 9 (8): 3 http://www.journalofvision.org/content/9/8/999, doi:10.1167/9.8.999. [Abstract] [Google Scholar]
- Joish V. N., Malone D. C., Miller J. M. (2003). A cost-benefit analysis of vision screening methods for preschoolers and school-age children. Journal of American Association for Pediatric Ophthalmology, 7 (4), 283–290 [DOI] [PubMed] [Google Scholar]
- Joubert S., Felician O., Barbeau E., Sontheimer A., Barton J. J., Ceccaldi M., Poncet M. (2003). Impaired configurational processing in a case of progressive prosopagnosia associated with predominant right temporal lobe atrophy. Brain , 126 (11), 2537–2550 [DOI] [PubMed] [Google Scholar]
- Kirwan C., O'Keefe M. (2008). Higher order aberrations in children with amblyopia. Journal of Pediatric Ophthalmology and Strabismus, 45 (2), 92–96 [DOI] [PubMed] [Google Scholar]
- Kooi F. L., Toet A., Tripathy S. P., Levi D. M. (1994). The effect of similarity and duration on spatial interaction in peripheral vision. Spatial Vision , 8 (2), 255–279 [DOI] [PubMed] [Google Scholar]
- Korte W. (1923). Über die Gestaltauffassung im indirekten Sehen. Zeitschrift für Psychologie , 93, 17–82 [Google Scholar]
- Kwon M., Legge G. E., Dubbels B. R. (2007). Developmental changes in the visual span for reading. Vision Research, 47 (22), 2889–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham K., Whitaker D. (1996). Relative roles of resolution and spatial interference in foveal and peripheral vision. Ophthalmic and Physiological Optics, 16 (1), 49–57 [PubMed] [Google Scholar]
- Leek C. E., Patterson C., Paul M. A., Rafal R., Cristino F. (2012). Eye movements during object recognition in visual agnosia. Neuropsychologia, 50 (9), 2142–2153 [DOI] [PubMed] [Google Scholar]
- Levi D. M. (1991). Spatial vision in amblyopia. In Regan D. (Ed.), Spatial Vision (pp 212–238) London: Macmillan Press; [Google Scholar]
- Levi D. M. (2008). Crowding—An essential bottleneck for object recognition: A mini-review. Vision Research , 48 (5), 635–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi D. M., Carkeet A. (1993). Amblyopia: A consequence of abnormal visual development. In Simons K. (Ed.), Early visual development, normal and abnormal (pp 391–408) Oxford, UK: Oxford University Press; [Google Scholar]
- Levi D. M., Carney T. (2009). Crowding in peripheral vision: Why bigger is better. Current Biology , 19 (23), 1988–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi D. M., Carney T. (2011). The effect of flankers on three tasks in central, peripheral, and amblyopic vision. Journal of Vision, 11 (1): 3 1–23, http://www.journalofvision.org/content/11/1/10, doi:10.1167/11.1.10. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Levi D. M., Hariharan S., Klein S. A. (2002. a). Suppressive and facilitatory spatial interactions in amblyopic vision. Vision Research, 42 (11), 1379–1394 [DOI] [PubMed] [Google Scholar]
- Levi D. M., Hariharan S., Klein S. A. (2002b). Suppressive and facilitatory spatial interactions in peripheral vision: Peripheral crowding is neither size invariant nor simple contrast masking. Journal of Vision , 2 (2): 3 167–177, http://www.journalofvision.org/content/2/2/3, doi:10.1167/2.2.3. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Levi D. M., Klein S. (1982). Hyperacuity and amblyopia. Nature, 298 (5871), 268–270 [DOI] [PubMed] [Google Scholar]
- Levi D. M., Klein S. A. (1985). Vernier acuity, crowding and amblyopia. Vision Research, 25 (7), 979–991 [DOI] [PubMed] [Google Scholar]
- Levi D. M., Klein S. A. (1990. a). Equivalent intrinsic blur in amblyopia. Vision Research, 30 (12), 1995–2022 [DOI] [PubMed] [Google Scholar]
- Levi D. M., Klein S. A. (1990. b). Equivalent intrinsic blur in spatial vision. Vision Research, 30 (12), 1971–1993 [DOI] [PubMed] [Google Scholar]
- Levi D. M., Klein S. A., Aitsebaomo A. P. (1985). Vernier acuity, crowding and cortical magnification. Vision Research, 25 (7), 963–977 [DOI] [PubMed] [Google Scholar]
- Levi D. M., Klein S. A., Hariharan S. (2002). Suppressive and facilitatory spatial interactions in foveal vision: Foveal crowding is simple contrast masking. Journal of Vision , 2 (2): 3 140–166, http://www.journalofvision.org/content/2/2/2, doi:10.1167/2.2.2. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Levi D. M., Song S., Pelli D. G. (2007). Amblyopic reading is crowded. Journal of Vision, 7 (2): 3 1–17, http://www.journalofvision.org/content/7/2/21, doi:10.1167/7.2.21. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Levi D. M., Waugh S. J., Beard B. L. (1994). Spatial scale shifts in amblyopia. Vision Research, 34 (24), 3315–3333 [DOI] [PubMed] [Google Scholar]
- Levi D. M., Yu C., Kuai S. G., Rislove E. (2007). Global contour processing in amblyopia. Vision Research, 47 (4), 512–524 [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R. W., Klein S. A., Levi D. M. (2008). Prolonged perceptual learning of positional acuity in adult amblyopia: Perceptual template retuning dynamics. The Journal of Neuroscience , 28 (52), 14223–14229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loshin D. S., Levi D. M. (1983). Suprathreshold contrast perception in functional amblyopia. Documenta Ophthalmologica , 55 (3), 213–236 [DOI] [PubMed] [Google Scholar]
- Manny R. E., Fern K. D., Loshin D. S. (1987). Contour interaction function in the preschool child. American Journal of Optometry and Physiological Optics, 64 (9), 686–692 [DOI] [PubMed] [Google Scholar]
- McGraw P. V., Winn B. (1993). Glasgow acuity cards: A new test for the measurement of letter acuity in children. Ophthalmic and Physiological Optics , 13 (4), 400–404 [DOI] [PubMed] [Google Scholar]
- McKee S. P., Levi D. M., Movshon J. A. (2003). The pattern of visual deficits in amblyopia. Journal of Vision, 3 (5): 3 380–405, http://www.journalofvision.org/content/3/5/5, doi:10.1167/3.5.5. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Mendez M. F., Shapira J. S., Clark D. G. (2007). “Apperceptive” alexia in posterior cortical atrophy. Cortex , 43 (2), 264–270 [DOI] [PubMed] [Google Scholar]
- Morad Y., Werker E., Nemet P. (1999). Visual acuity tests using chart, line, and single optotype in healthy and amblyopic children. J AAPOS: The official publication of the American Association for Pediatric Ophthalmology and Strabismus , 3 (2), 94–97 [DOI] [PubMed] [Google Scholar]
- Pelli D. G. (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision , 10 (4), 437–442 [PubMed] [Google Scholar]
- Pelli D. G., Bex P. (2013). Measuring contrast sensitivity. Special issue on testing vision. Vision Research , 90, 10–14, doi:10.1016/j.visres.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli D. G., Palomares M., Majaj N. J. (2004). Crowding is unlike ordinary masking: Distinguishing feature integration from detection. Journal of Vision , 4 (12): 3 1136–1169, http://www.journalofvision.org/content/4/12/12, doi:10.1167/4.12.12. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Pelli D. G., Song S., Levi D. M. (2011). Improving the screening of children for amblyopia. Journal of Vision , 11 (11): 3 http://www.journalofvision.org/content/11/11/411, doi:10.1167/11.11.411. [Abstract] [Google Scholar]
- Pelli D. G., Tillman K. A. (2008). The uncrowded window of object recognition. Nature Neuroscience, 11 (10), 1129–1135, http://www.nature.com/neuro/journal/v11/n10/index.html#pe [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli D. G., Tillman K. A., Freeman J., Su M., Berger T. D., Majaj N. J. (2007). Crowding and eccentricity determine reading rate. Journal of Vision, 7 (2): 3 1–36, http://www.journalofvision.org/content/7/2/20, doi:10.1167/7.2.20. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Polat U., Bonneh Y., Ma-Naim T., Belkin M., Sagi D. (2005). Spatial interactions in amblyopia: Effects of stimulus parameters and amblyopia type. Vision Research , 45 (11), 1471–1479 [DOI] [PubMed] [Google Scholar]
- Regan D., Giaschi D. E., Kraft S. P., Kothe A. C. (1992). Method for identifying amblyopes whose reduced line acuity is caused by defective selection and/or control of gaze. Ophthalmic and Physiological Optics, 12 (4), 425–432 [PubMed] [Google Scholar]
- Riddoch M. J., Humphreys G. W. (1987). A case of integrative visual agnosia. Brain , 110, 1431–1462 [DOI] [PubMed] [Google Scholar]
- Roorda A., Romero-Borja F., Donnelly W. III,, Queener H. (2002). Adaptive optics scanning laser ophthalmoscopy. Optics Express , 10 (9), 405–412 [DOI] [PubMed] [Google Scholar]
- Siderov J., Waugh S. J., Bedell H. E. (2013). Foveal contour interaction for low contrast acuity targets. Vision Research , 77, 10–13 [DOI] [PubMed] [Google Scholar]
- Simmers A. J., Gray L. S., Spowart K. (1997). Screening for amblyopia: A comparison of paediatric letter tests. British Journal of Ophthalmology , 81 (6), 465–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K. (1983). Visual acuity norms in young children. Survey of Ophthalmology, 28 (2), 84–92 [DOI] [PubMed] [Google Scholar]
- Simons K. (1996). Preschool vision screening: Rationale, methodology and outcome. Survey of Ophthalmology , 41 (1), 3–30 [DOI] [PubMed] [Google Scholar]
- Simons K. (2005). Amblyopia characterization, treatment, and prophylaxis. Survey of Ophthalmology, 50 (2), 123–166 [DOI] [PubMed] [Google Scholar]
- Simons K., Moss A. (1981). A dynamic random dot stereogram-based system for strabismus and amblyopia screening of infants and young children. Computers in Biology and Medicine, 11 (1), 33–46 [DOI] [PubMed] [Google Scholar]
- Snellen H. (1866). Test-types for the determination of the acuteness of vision (4th ed.). London: Williams & Norgate; [Google Scholar]
- Snowdon S. K., Stewart-Brown S. L. (1997). Preschool vision screening. Health Technology Assessment, 1 (8), i–iv, 1–83 [PubMed] [Google Scholar]
- Sternberg S. (2003). Process decomposition from double dissociation of subprocesses. Cortex , 39, 180–182 [DOI] [PubMed] [Google Scholar]
- Strappini F., Pelli D. G., Di Pace E., Martelli M. (submitted). Agnosic vision is crowded. Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasburger H. (2005). Unfocused spatial attention underlies the crowding effect in indirect form vision. Journal of Vision, 5 (11): 3 1024–1037, http://www.journalofvision.org/content/5/11/8, doi:10.1167/5.11.8. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Strasburger H., Harvey L. O. Jr.,, Rentschler I. (1991). Contrast thresholds for identification of numeric characters in direct and eccentric view. Perception & Psychophysics, 49 (6), 495–508 [DOI] [PubMed] [Google Scholar]
- Stuart J. A., Burian H. M. (1962). A study of separation difficulty. Its relationship to visual acuity in normal and amblyopic eyes. American Journal of Ophthalmology , 53, 471–477 [PubMed] [Google Scholar]
- Thomas-Decortis G. (1959). [Angular visual acuity and morphoscopic visual acuity in amblyopia ex anopsia.]. The Bulletin of the Belgian Society of Ophthalmology , 123, 488–499 [PubMed] [Google Scholar]
- Toet A., Levi D. M. (1992). The two-dimensional shape of spatial interaction zones in the parafovea. Vision Research, 32 (7), 1349–1357 [DOI] [PubMed] [Google Scholar]
- Tommila V. (1972). A new chart for testing line acuity in amblyopia. Acta Ophthalmologica (Copenhagen), 50 (4), 565–569 [DOI] [PubMed] [Google Scholar]
- Warrington E. K., Plant G. T., James M. (2001). Cortical Vision Screening Test (CORVIST). Retrieved from [Link] [Google Scholar]
- Watt R. J., Hess R. F. (1987). Spatial information and uncertainty in anisometropic amblyopia. Vision Research, 27 (4), 661–674 [DOI] [PubMed] [Google Scholar]
- Watt R. J., Morgan M. J. (1984). Spatial filters and the localization of luminance changes in human vision. Vision Research, 24 (10), 1387–1397 [DOI] [PubMed] [Google Scholar]
- Whitney D., Levi D. M. (2011). Visual crowding: a fundamental limit on conscious perception and object recognition. Trends in Cognitive Sciences, 15 (4), 160–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick B., Schor C. M. (1984). A comparison of the Snellen chart and the S-chart for visual acuity assessment in amblyopia. Journal of the American Optometric Association, 55 (5), 359–361 [PubMed] [Google Scholar]