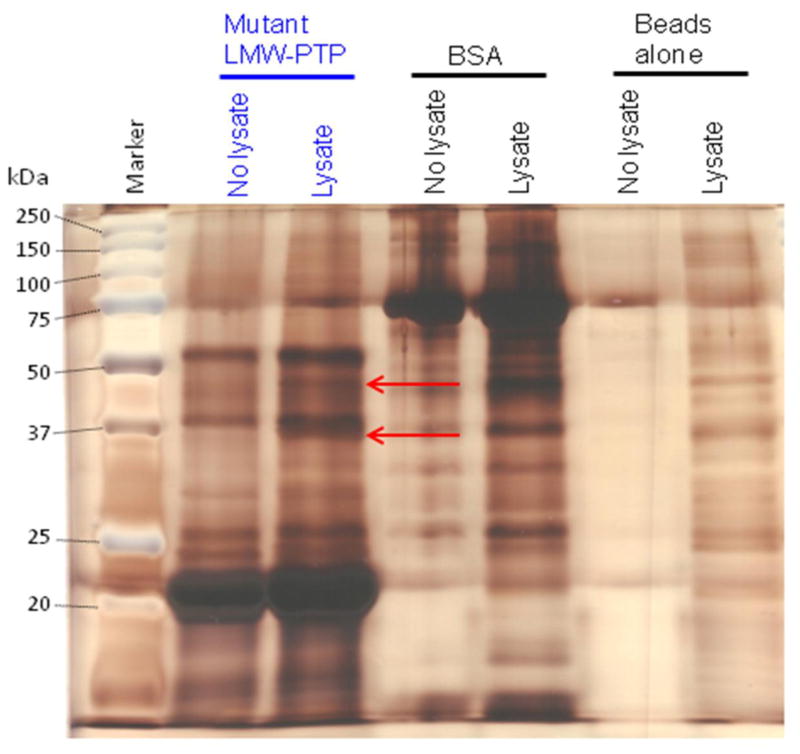
Fig. 5. A representative silver-stained gel of pulldowns using substrate-trapping mutant LMW-PTP or BSA protein coupled to Affi-Gel 15 beads and submitted for tryptic digest and mass spectrometry analysis.
Recombinant Cys to Ser mutant LMW-PTP or BSA were coupled to Affi-Gel 15 beads, or beads alone (mock-attached) were incubated with buffer only. Beads were incubated with lysate from HM1:IMSS amebae pre-treated with pervanadate (Lysate) or with lysis buffer alone (No lysate). Samples were subjected to SDS-PAGE and silver-stained. There are two unique bands (red arrows) of ~37 and ~45 kDa in the Affi-Gel 15 beads with coupled mutant LMW-PTP incubated with amebic lysate. These bands were submitted for tryptic digest and mass spectrometry analysis. Some of the mutant LMW-PTP was released from the Affi-Gel 15 beads when samples were boiled before loading, and ran at ~22 kDa rather than at 17 kDa. This is likely due to the large amount of protein that had been attached to the Affi-Gel 15 beads. The BSA also ran at a larger apparent size, about ~75 kDa, rather than its expected ~66 kDa for likely the same reason.