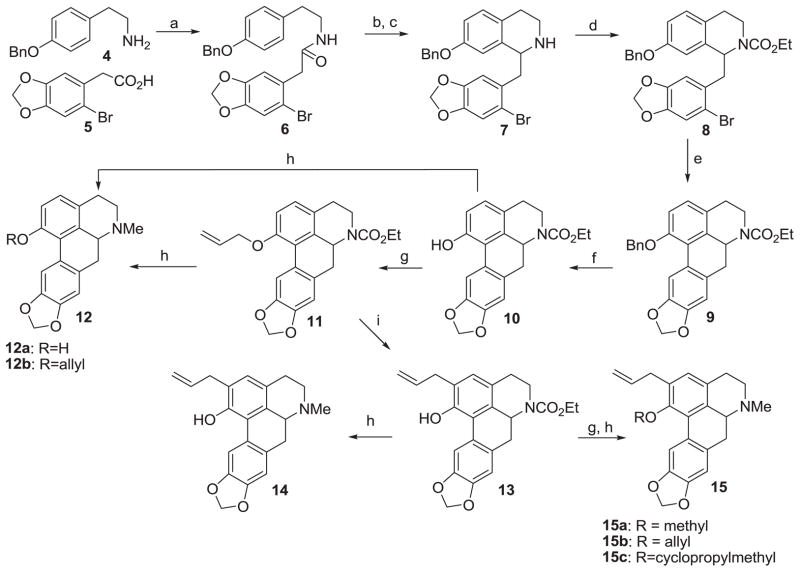
Scheme 1.
Synthesis of ring A analogs
Reagents and conditions: (a) 1,1′-carbonyldiimidazole (CDI), THF, 0 °C - rt, 5 h, 80% ; (b) trifluoromethanesulfonic acid, pyridine, DCM, 0 °C - rt, 4 h; (c) NaBH4, MeOH, 0 °C, 2 h, 88% over two steps; (d) Ethyl chloroformate, K2CO3, DCM, rt, 3 h, 85% ; (e) Pd(OAc)2, di-tert-butyl(methyl)phosphonium tetrafluoroborate, K2CO3, (CH3)3CCOOH, DMSO, 135 °C, microwaves, 6 min, 50% ; (f) H2/Pd, rt, 8 h, 95% ; (g) alkyl bromide, KI, K2CO3, acetone, 70 °C, 6 h, 60–70% ; (h) LAH, THF, 0 °C, 10 h, 50–60% ; (i) N,N-diethylaniline, 215 °C, microwaves, 6 min, 90%