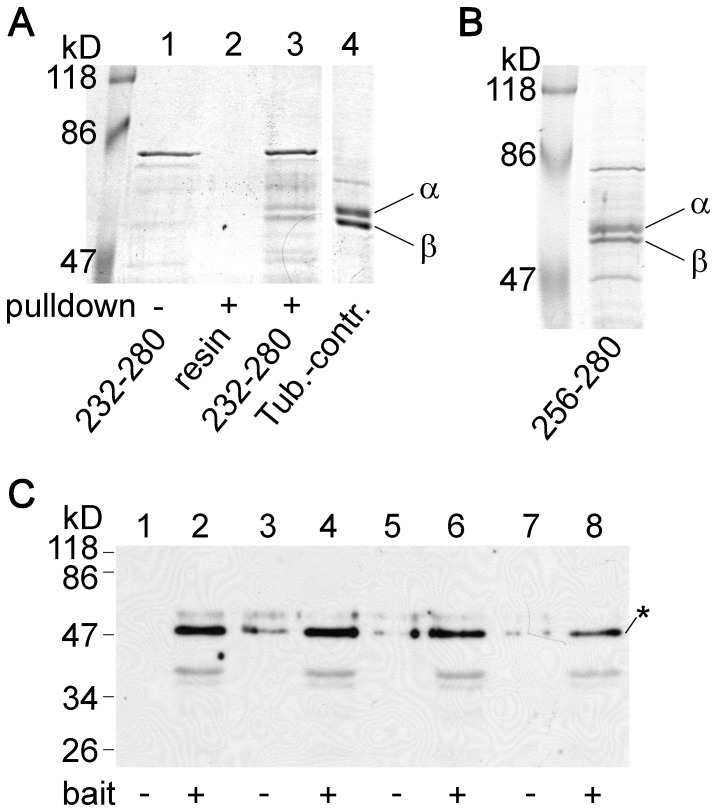
Figure 7. Tubulin interacting with the cytoplasmic loop of TRESK migrates as a double band on SDTHS-PAGE gel.
A. The proteins from the pull-down assay with fragment 232–280 of human TRESK were analyzed by SDS-PAGE in the presence of STS and SHS. Under these special conditions, the band split into a doublet of α and β tubulins (lane 3), although the same protein migrated as a single band on normal SDS-PAGE gels (see in Fig. 3.B lane 5 or Fig. 3.C. lane 4). The double band could be detected neither in the bait protein preparation (lane 1) nor in the control pull-down assay with glutathione agarose (lane 2). B. Similar splitting of the band was observed on SDTHS-PAGE gels in the case of tubulin pulled down with fragment 256–280 of human TRESK. C. Western blot experiment with monoclonal anti-tubulin β3 antibody was performed from four pairs of independent pull-down assays. The analyzed proteins were pulled down from mouse brain cytosol with the GST fusion protein containing residues 174–280 of human TRESK (even lanes, bait +) or with glutathione agarose (odd lanes, bait −). The anti-tubulin β3 antibody specifically labeled the tubulin bands (indicated with an asterisk). Densitometry and statistical analysis were performed as detailed in Fig. S3.