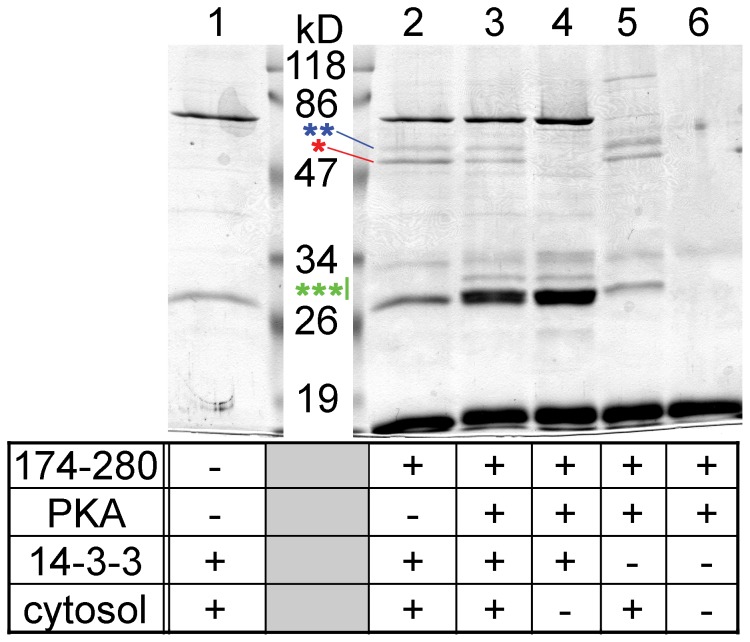
Figure 8. The adaptor protein 14-3-3 competes with tubulin for the binding to TRESK.
TRESK-loop-His8 (amino acids 174–280 of the human channel; immobilized on Ni-NTA resin) was (lane 3) or was not (lane 2) phosphorylated with protein kinase A (PKA). The bait was incubated with supernatant from E. coli expressing 14-3-3η adaptor protein without fusion tag. Subsequently, mouse brain cytosol was added, and pull-down assay was performed. The binding of 14-3-3 to the phosphorylated bait is apparent in the 30 kD range (as indicated with a green triple asterisk; compare lane 3 to 2). Calcineurin (blue double asterisk) interacted identically with the non-phosphorylated (lane 2) and the 14-3-3-preloaded bait (lane 3). In contrast, more tubulin (indicated with a red asterisk) was pulled down by TRESK-loop-His8 with no bound 14-3-3 (lane 2) than by the bait preloaded with the adaptor protein (lane 3). Several control reactions were also performed (as indicated in the table below the gel) to demonstrate that the bands corresponding to tubulin and calcineurin were of cytosolic origin (lanes 2 and 3 vs. 4), to determine the source of 14-3-3 binding to TRESK-loop-His8 (bacterial supernatant or brain cytosol, lane 3 vs. 5), to identify the bands belonging to the bait and PKA preparations (lane 6) and to illustrate the non-specific interactions of Ni-NTA resin in this experiment (lane 1).