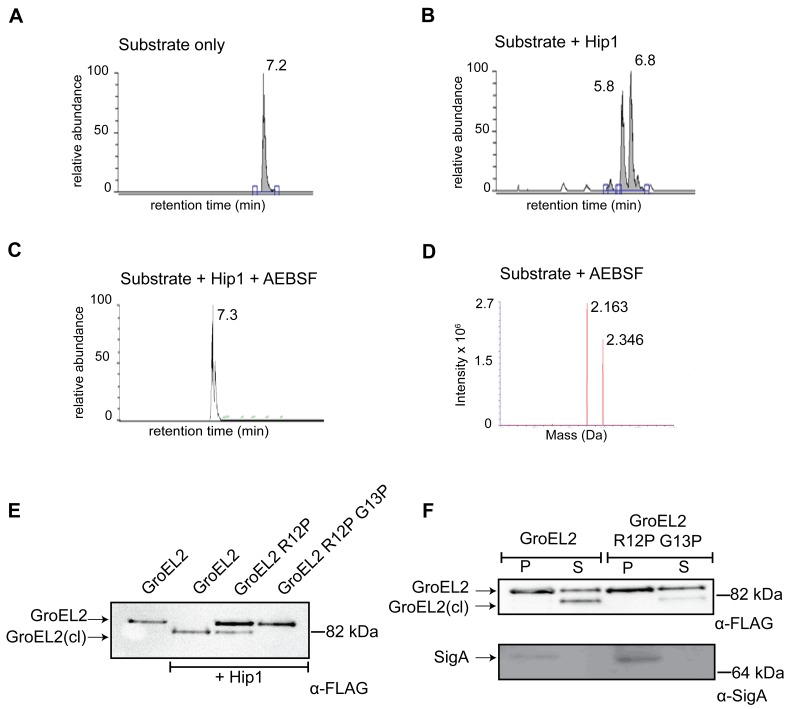
Figure 4. GroEL2 is cleaved in vitro and in vivo by Hip1.
(A) LC/MS/MS showing elution of the uncleaved GroEL2 N-terminal peptide (m/z = 2163). (B) LC peak profile of GroEL2 N-terminal peptide incubated with Hip1 for 18 hours at 25°C. Two abundant product fragments with m/z = 1122 and 757 indicate cleavages between Thre3 and Ile4, as well as between Arg12 and Gly13. (C) AEBSF inhibits Hip1 cleavage of the GroEL2 peptide. (D) Mass spectrometry analysis of the 7.3 min peak in (C) indicates the predominant species is uncleaved GroEL2. The second peak corresponds to substrate sulfonated by AEBSF at threonine. (E) Determining cleavage site of Mtb GroEL2 using M. smegmatis. Culture supernatants from M. smegmatis strains expressing Mtb GroEL2-FLAG, GroEL2 (R12P)-FLAG or GroEL2 (R12P G13P)-FLAG were subjected to cleavage by recombinant Hip1 for 24 hours at 37°C and analyzed by Western blot. (F) Cleavage site of GroEL2 in Mtb. GroEL2-FLAG or GroEL2 (R12P G13P)-FLAG mutant were expressed in Mtb H37Rv. Protein extracts corresponding to the pellet (P) and supernatant (S) fractions of those strains were prepared, and analyzed by Western blot with anti-FLAG antibody (to detect GroEL2) and anti-SigA antibody (to detect the sigma 70 subunit of RNA polymerase). Data (A–F) are shown as one representative experiment from three independent experiments.