FIGURE 5.
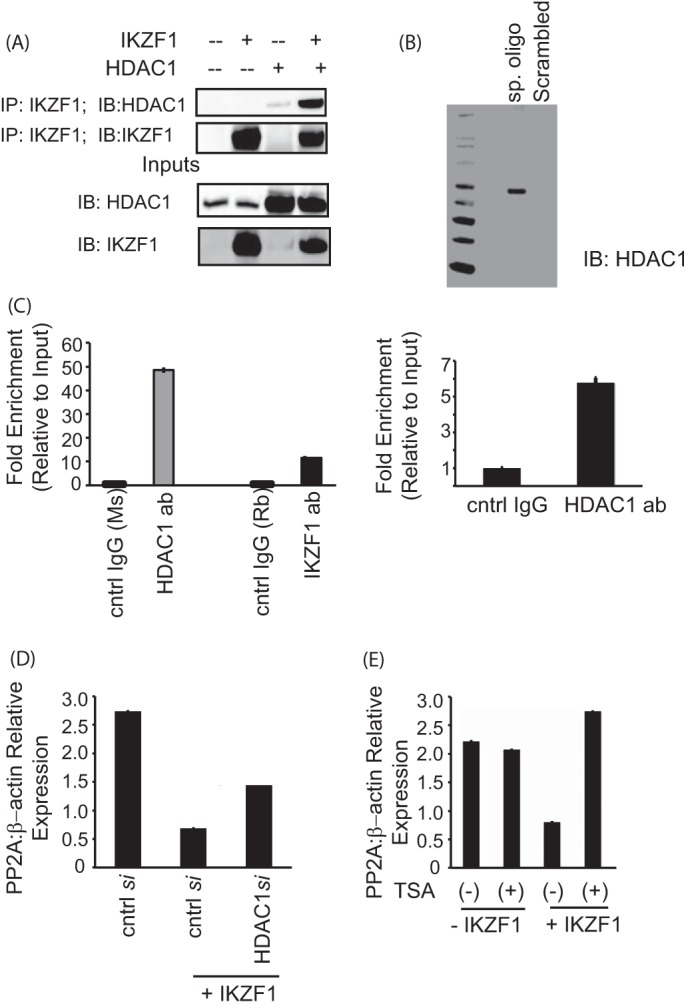
Ikaros-mediated repression of PP2A expression is dependent on HDAC1. A, coimmunoprecipitation (IP) assay showing the binding of Ikaros with HDAC1. 293T cells were transfected with the various combinations of plasmids using Lipofectamine 2000. Twenty-four hours after transfection, the cells were lysed, and the supernatants were incubated with Ikaros antibody for 2 h at 4 °C. After the preincubation with the antibody, agarose A/G beads were added to each sample and incubated overnight at 4 °C. The immunoprecipitates were subsequently run on a gel, transferred to a PVDF membrane, and blotted for the indicated proteins. The saved inputs were also run on the gel to confirm equal expression of proteins in all samples. IB, immunoblot. B, a biotin-conjugated, intron-specific oligo (sp. oligo) or a random control oligo were used in pulldown assays with Jurkat cell nuclear extracts. The eluates were run on a gel and probed with anti-HDAC1 antibody. C, reporter ChIP (left panel) and ChIP (right panel) in 293T and primary T cells, respectively. 293T cells (left panel) were transfected with the reporter in combination with the Ikaros and HDAC1 expression plasmids using Lipofectamine 2000. Twenty-four hours after transfection, cells were collected, and a ChIP assay was performed using the MAGnify ChIP kit. For ChIP with endogenous protein in primary T cells (right panel), 5 million freshly isolated primary T cells were used for each antibody (ab)/sample. The ChIP assay was carried out using the MAGnify ChIP kit. The region spanning the specific intron site was amplified by quantitative PCR and normalized to the values obtained from the input DNA. The graph shows mean ± S.D. of three observations. cntrl, control; Rb, rabbit; Ms, mouse. D, T cells were transfected with control siRNA or 10 nm HDAC1-specific siRNA with or without cotransfection of the Ikaros plasmid using AMAXA. 72 h after transfection, cells were harvested and subjected to real-time PCR analysis. The housekeeping gene β-actin was used for normalization. E, primary T cells were transfected with the Ikaros plasmid using AMAXA. Twenty-four hours after transfection, the cells were treated with 100 ng/ml trichostatin A (TSA) for 12 h. The same volume of dimethyl sulfoxide was used as in the vehicular control. The cells were harvested, and real-time PCR was carried out. The housekeeping gene β-actin was used for normalization.
