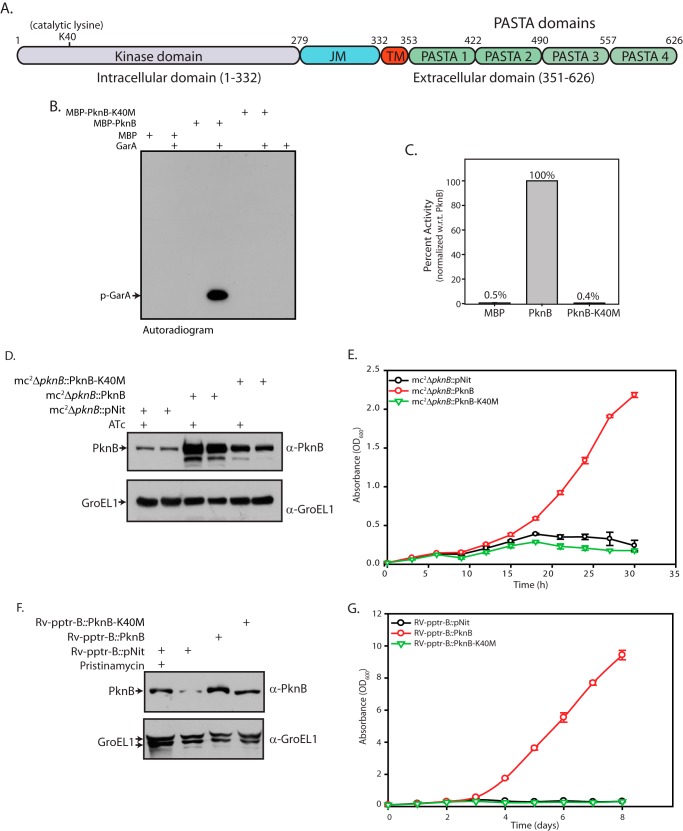
FIGURE 3.
Kinase activity of PknB is essential for its functionality. A, schematic representation of PknB depicting the various domains and critical residues. B, in vitro kinase assays were carried out using 80 nm MBP or PknB or PknB-K40M, 3.33 μm GarA, 10 μm ATP, and 10 μCi [γ-32P]ATP. The band corresponding to the phosphorylated GarA (p-GarA) is indicated. C, bands corresponding to phospho-GarA were excised from the gel and quantified by liquid scintillation counting in three independent experiments. The activity was calculated as cpm in phospho-GarA/min/μm enzyme. Activity of PknB was normalized to 100% in each experiment, and the percentage of activity in other samples was calculated with respect to (w.r.t.) PknB activity. The results were plotted with percentage of activity on the y axis and the samples on the x axis in the form of histograms. D, mc2ΔpknB strain was transformed with pNit vector, pNit-PknB, or pNit-PknB-K40M constructs. Cultures grown to A600 ∼0.8 in the presence of inducer (ATc) were washed twice, and fresh cultures were seeded at an initial A600 of 0.2. Cultures were grown in the presence or absence of ATc as indicated above for 6 h and WCLs were resolved and subjected to Western blotting with α-PknB and α-GroEL1 antibodies. E, growth pattern analysis of mc2ΔpknB transformants grown in the absence of ATc. F, Rv-pptr-B transformants grown to A600 ∼0.8 in the presence of inducer pristinamycin 1A were washed twice, and fresh cultures were seeded at an initial A600 of 0.2. Cultures were grown for 4 days in the presence or absence of pristinamycin as indicated above, and WCLs were resolved from transformant strains and subjected to Western blotting with α-PknB and α-GroEL1 antibodies. G, growth pattern analysis of Rv-pptr-B transformants in the absence of pristinamycin. All of the cultures were seeded at an initial A600 of 0.1. Error bars, S.E.