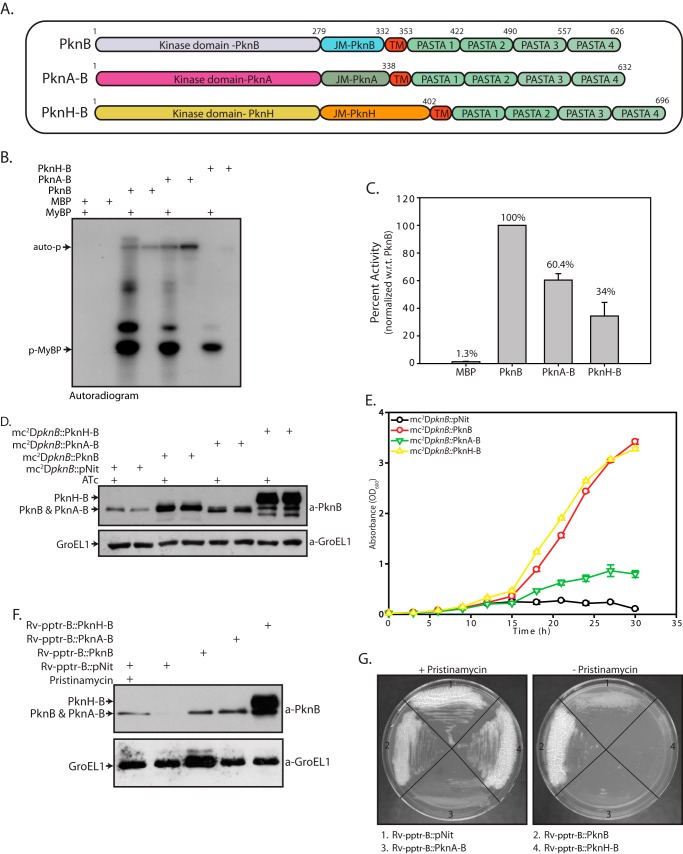
FIGURE 7.
Intracellular domain of M. tuberculosis has a specific and distinct indispensable role. A, schematic representation of PknB chimeric proteins. B, MBP-tagged PknB, PknA-B, and PknH-B proteins were purified, and in vitro kinase assays were carried out using 25 nm MBP, PknB, PknA-B, or PknH-B; 6.25 μm MyBP; and 10 μCi of [γ-32P]ATP. Auto-p, autophosphorylation of respective kinases. p-MyBP, phosphorylated MyBP. Samples were resolved on 12% SDS-PAGE and autoradiographed. C, bands corresponding to phospho-MyBP were excised from the gel and quantified by liquid scintillation counting in three independent experiments. The activity was calculated as cpm of phospho-MyBP/min/μm enzyme. The activity of PknB was normalized to 100% in each experiment, and the percentage of activity in other samples was calculated with respect to (w.r.t.) PknB activity. The results were plotted with percentage of activity on the y axis and the samples on the x axis. D, mc2ΔpknB strain was transformed with pNit, pNit-PknB, pNit-PknA-B, or pNit-PknH-B constructs was seeded at A600 of 0. 2 and grown in the presence or absence of ATc. WCLs were resolved and probed with α-PknB and α-GroEL1 antibodies. E, growth analysis of mc2ΔpknB transformants seeded at A600 of 0.02 in the absence of inducer ATc. F, Rv-pptr-B transformed with pNit, pNit-PknB, pNit-PknA-B, or pNit-PknH-B was seeded at an A600 of 0.2. WCLs prepared in the presence or absence of pristinamycin (as indicated) were resolved and probed with α-PknB and α-GroEL1 antibodies. G, Rv-pptr-B transformants were streaked on 7H10 agar plates with or without pristinamycin. Error bars, S.E.