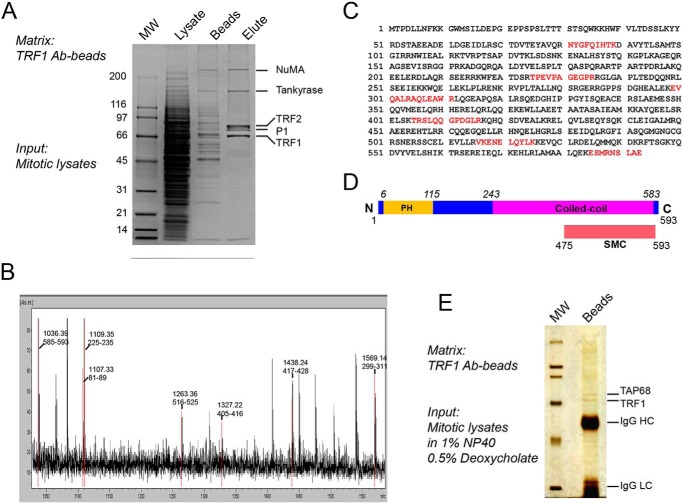
FIGURE 1.
Identification of a novel TRF1-binding protein, TAP68. A, mitotic HeLa cell extracts were applied to a TRF1 peptide antibody affinity column. After binding, columns were extensively washed, and bound proteins were eluted and separated by SDS-PAGE. The indicated proteins were extracted from the gel and digested with trypsin, and the amino acid sequence of the peptides was determined by MALDI-TOF and LC-MS/MS mass spectrometry. P1 is TAP68. B, protein band of P1 was extracted from the acrylamide gel and digested with trypsin, and the resulting peptide fragments were subjected to mass spectrometric analysis. C, P1 peptides match a previously uncharacterized protein with a 593-amino acid open reading frame of unknown function (AAH03618). D, schematic representation of the TAP68 domain organization predicted by NCBI's on-line conserved domain search tool. E, TRF1-TAP68 interaction was confirmed by immunoprecipitation assay. The anti-TRF1 antibody binding on protein A/G beads was incubated with mitotic cell lysate prepared in high stringency lysis buffer containing 1% Nonidet P-40 and 0.5% deoxycholate. Under this condition, TAP68 remained firmly bound to TRF1, whereas the majority of other TRF1-binding proteins, such as TRF2 and tankyrase, were absent from the co-immunoprecipitates.