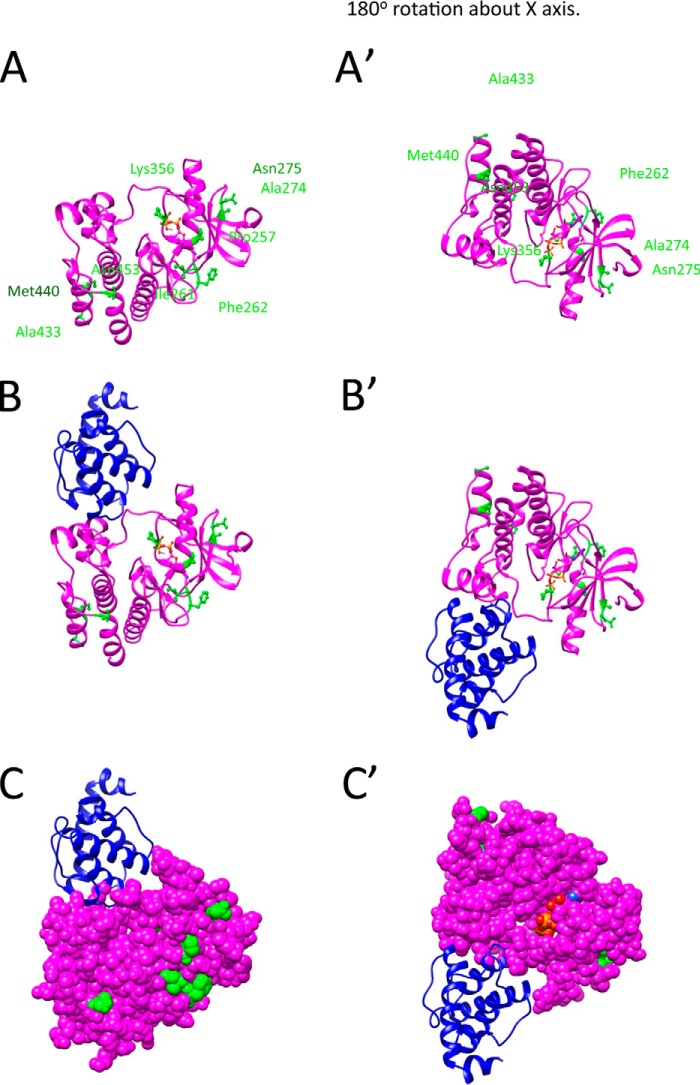
FIGURE 2.
Homology model of PAT-4 with location of suppressor mutations. A, model of PAT-4 based on the crystal structure of human ILK is shown as a magenta ribbon. The positions of PAT-4 suppressor mutations are shown in green balls and sticks. ATP is shown colored by atom color in stick form. The view on the right was created by rotating the view on the left by 180° around the x axis. Note that the suppressor mutations cluster in two regions on the surface of PAT-4. Pro-257, Ile-261, Phe-262, Ala-274, Asn-275, and Lys-356 cluster near the edge of the β-sheet and helix 1 in the N-terminal domain. Ala-433, Met-440, and Asn-453 cluster at the C-terminal helices. B, two views show the homology model of PAT-4 kinase with α-parvin (PAT-6 in nematodes). PAT-4 was substituted for human ILK in the human ILK·α-parvin complex (Protein Data Bank ID code 3KMW) (24). PAT-4 is shown in magenta, and α-parvin (PAT-6) is shown in dark blue. Note that α-parvin does not overlap or cover either cluster of the suppressor mutations. They are available to form another binding surface. C, two views show of the same complex as in B with PAT-4 in space fill mode. The suppressor mutations are shown in green. Note that a majority of the suppressor mutations (Pro-257, Ile-261, Phe-262, Ala-274, Asn-275, Lys-356, and Asn-453) are on the far surface from α-parvin (PAT-6).