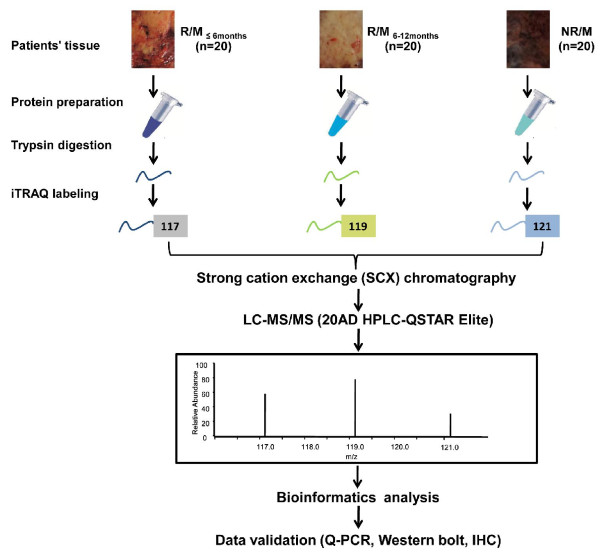
Figure 5.
Outline of the iTRAQ based quantitative proteomic strategy. iTRAQ labeling was carried out by using tumor tissues from the patients who had recurrence/metastasis within 6 months after operation (R/M≤6months group, n = 20); the patients whose recurrence/metastasis occurred between 6 and 12 months after operation (R/M6-12months group, n = 20); and the patients who had no recurrence/metastasis within 2 years of operation (NR/M group, n = 20). Samples were digested by trypsin, and the peptides from R/M≤6months, R/M6-12months, and NR/M were labeled by iTRAQ reagents 117, 119 and 121, respectively. After labeling, peptides from all three samples were combined and fractionated by SCX chromatography. Each fraction was then analyzed by LC-MS/MS on a QSTAR Elite mass spectrometers. The identified potential interesting targets were further validated by Q-PCR, Western bolt and immunohistochemistry (IHC).