Abstract
HIV/AIDS has reached a pandemic level across the world with more than 33 million people who are living with HIV. In the United States, more than half a million people have been victims of AIDS. This study investigates the most vulnerable racial minority population (the African Americans) in the United States and the second least affected (the Caucasians) in order to predict the trends of the epidemic. A Markov chain analysis was used to model the progression of the disease among vulnerable people, infective people and AIDS cases for the two races separately, based on the 2009 Centers of Disease Control and Prevention HIV/AIDS Surveillance Report. Based on the Markov model, our study predicts that the number of African American people living with AIDS diagnosis and HIV infection and dead due to HIV/AIDS will be 662.2, 1225.3 and 62.9 in 2015 and 794.9, 1566.5 and 79.2 in 2030, respectively. The number of Caucasian people living with AIDS diagnosis and HIV infection and dead due to HIV/AIDS will be 96.4, 160 and 6.5 in 2015 and 118.6, 206.9 and 8.3 in 2030, respectively. The numbers of deaths due to HIV/AIDS are quite stable over the years in both the races. There is an increasing trend in the number of people living with HIV infection and AIDS diagnosis in Caucasians compared with African Americans. The absolute number of Caucasians living with AIDS diagnosis and HIV infection is quite smaller compared with African Americans. The results reveal discrepancy in HIV infection, AIDS diagnosis and deaths due to HIV/AIDS among the African Americans and the Caucasians races. There is a need for interventions focusing on HIV/AIDS prevention and management, optimum resource allocation and development of antiAIDS campaigns to reduce the infection rate.
Keywords: HIV/AIDS virus progression, Markov model, race discrepancy, forecast, HIV/AIDS public healthcare
The human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) has become an epidemic since it was first identified in 1981[1]. In December 2010, it was estimated that 33 million people worldwide were living with HIV/AIDS and the number of deaths caused by HIV/AIDS were over 35 million[2]. In the United States, the number of people who were living with HIV/AIDS was estimated to be 1.2 million by the end of 2009, and it is estimated that about 50 000 new cases occur annually[3].
African Americans continue to experience severe burden of HIV, compared with other races and ethnicities. African Americans represent approximately 14% of the US population, but accounted for an estimated 44% of new HIV infections in 2009 and 46% of people living with HIV infection in 2008. In 2009, the estimated rate of new HIV infections among African American men was six and a half times as high as that of Caucasian men. In the same year, the estimated rate of new HIV infections among African American women was 15 times that of Caucasian women[4].
These racial disparities in HIV/AIDS may be attributed to many different factors. African Americans carry more risk factors of HIV/AIDS transmission. They tend to have sex with the people of the same race[4]. In the risk group of men who have sex with men (MSM), young Blacks shared the largest proportion compared with any other age and race/ethnicity[4]. Compared with all the racial minorities in the US, African Americans also experience the highest prevalence of sexually transmitted infections (STDs)[4]. Moreover, 20% of the infected people in the US were unaware of their HIV/AIDS status[3]. African Americans tend to have more negative perceptions regarding HIV diagnosis tests[3]. This may increase delayed diagnosis, delayed treatment, under-recognition and may further worsen the transmission. Other factors associated with racial disparities in HIV/AIDS include socioeconomic, cultural, religious and social influences that contribute to stigma and discrimination. Some of the racial disparities in HIV/AIDS have decreased over time. However, racial differences still exists with regards to HIV/AIDS disease management in US.
Although current treatments, such as highly active antiretroviral therapy (HAART), have successfully improved the survival and life expectancies in HIV/AIDS population, the epidemic continues to affect newer populations and remain prevalent among those who have already experienced this epidemic[3]. Therefore, it is necessary to focus efforts on both treatment advancement and disease prevention measures to reduce racial disparities. Our study will utilise a Markov chain model to predict the trends of the epidemic among the African Americans and Caucasians separately. Forecasting the progression of HIV/AIDS spread plays an important role in controlling disease transmission and alleviating health disparities. Given the growing threat of limited resources in the society, especially in the health care system and public health system, the projection of the future epidemic can help us to optimise resource allocation and design efficient, economical, timely health policies targeting the high risk populations and high prevalence areas.
A Markov process is a type of stochastic processes in which a system changes in a random manner between different states, at regular or irregular intervals[5]. Markov chain modelling has been applied to a number of studies in the medical field over the years to predict and estimate random or uncertain events associated with specific probabilities of occurrence. Some practical applications have been observed in analysis of genetics, particularly in sickle cell anaemia, determining efficacy of noninsulin-dependent diabetes in a population of patients[6], predicting outcomes of dialysis treatment/kidney transplants in patients[7] and analysing longitudinal disease progression for liver cancer[8], breast cancer[9], bronchiolitis obliterans syndrome[10] and Alzheimer's disease[11], among others.
With the aim of contributing to the epidemiological surveillance of HIV/AIDS, Bature et al.[12] described a Markov chain model that was used to track the movement of the virus from one generation to another in a period of 20 years. Sweeting et al.[13] applied multistate Markov modelling to explain the rate at which the Hepatitis C disease progresses. Observational data on 1306 patients in Trent, England was collected from 1991 to 2006 and used for the longitudinal study. Debanne et al.[14] developed a multivariate Markov chain model to project tuberculosis (TB) progression in the US from the 1980s to 2010 among different races in the country.
Although previous studies have modelled disease progress and transmission dynamics using Markov models[15,16,17,18], few have focused on epidemiological disease progression at a macro level of total populations through Markov models. The prediction of both incidence and prevalence of HIV/AIDS, made using the Markov model, will help in planning and calibrating adequate surveillance systems, as well as in allocating public health resources and in targeting intervention and treatment plans.
MATERIALS AND METHODS
In this study, the HIV/AIDS progression is assumed to follow a discrete time Markov chain with stationary transition probabilities, which is represented as: {X(t), t ∈ T} with time index set T ={0,1,…} and a finite state space S ={S1,S1,…,SN}. This model satisfies the following the Markovian property; P{X(t)=Si|X(t−2)=Sk,…} = P{X(t)=Si|X(t−)=Sj}(1) for all t and all possible states. This Markovian property means that the probability of the random variable X (t) being in state Si at time t depends only on the variable's state Si at time t − 1, but not on states at previous points in time. In other words, it does not depend on how the system has led to the current state. Thus, for a Markov process, the state of the process at a given time contains all the information about the past evolution of the process which is of use in predicting its future behaviour[5].
We assume that epidemiological disease progression in individuals at large follows the Markovian property. This assumption enables us to model and simulate the epidemic as a Markov chain through which HIV-infected individuals’ progress to AIDS and death over time. The Markov model can provide a useful tool to analyse or model a stochastic progression behaviour despite insufficient historical data points. Thus, we will use it in our study to predict future trends of HIV/AIDS progression so that health policies can be framed in advance to contain its associated costs and manage it.
Markov model analysis and verification:
The states to be modelled in this paper are S1: The rate of vulnerable people (V); S2: The rate of people with HIV diagnosis (H); S3: The rate of people with AIDS diagnosis (A); and S4: The rate of deaths from the HIV/AIDS virus (D) where these rates were computed by dividing the numbers of cases reported by the population for that year and multiplying by 100 000.
The states in fig. 1 represent four different stages of the progression of the disease among vulnerable, HIV infective, clinical AIDS persons and death. The transient states are S1, S2 and S3 while the recurrent absorbing state is S4. Transitions between these states will be modelled separately for the African Americans and Caucasians.
Fig. 1.
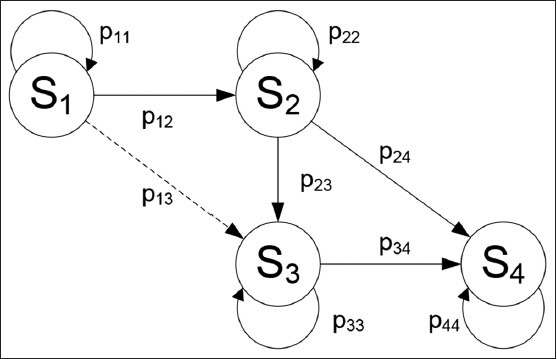
Transition diagram for the Markov Chain.
S (states) represents four different stages of the progression of the disease among vulnerable, HIV infective, clinical AIDS persons, and death. The transient states are S1, S2 and S3, while the recurrent absorbing state is S4. Transitions between these states will be modelled separately for the African Americans and Caucasians.
Note that the transition from state S1 to state S3 cannot occur clinically. However, the transition from state S1 to state S3 includes people whose diagnoses of HIV infection and AIDS were made at the same time or those whose AIDS diagnosis after a diagnosis of HIV infection was made within a year, because the time epoch of this model is one year (i.e. a census is taken every year). The transition from state S2 to state S3 includes HIV diagnosed people who eventually developed AIDS. The values of p24 and p34 represent the transition probability from HIV diagnosis and AIDS diagnosis to death, respectively.
The state probabilities of the states S2, S3 and S4 calculated from the above Markov model will be proportional to the rate of persons living with a diagnosis of HIV, persons living with an AIDS diagnosis and deaths with HIV/AIDS, respectively. The state probability information will be used to estimate transition probabilities and to validate the Markov models.
Data for HIV/AIDS:
The data used for this study was collected over a period of 4 years (2006–2009) from the Centers of Disease Control and Prevention (CDC) database and Prevention HIV/AIDS Surveillance Report 2009 from the 40 US states[3]. Data was given as the rate r per 100 000 people for each category. These rates were computed by dividing the numbers of cases reported by the population for that year and multiplying by 100 000. Table 1 displays the estimated rates for African Americans and Caucasians, respectively.
TABLE 1.
ESTIMATED RATES FOR AFRICAN AMERICANS AND CAUCASIANS IN THE YEAR 2006-2009
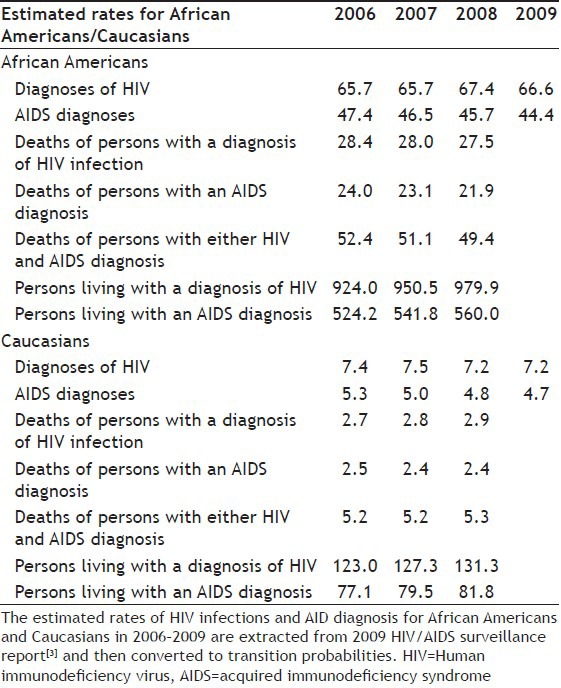
These rates were then converted to the respective transition probabilities. The calculation for transition matrices will be explained in the section below.
Markov model analysis:
In this section, we establish and analyse Markov models for the African Americans and Caucasians not only for a better understanding of the racial discrepancy among HIV/AIDS dynamic evolution, but also to discuss health policies and resource allocations with the projected HIV/AIDS behaviors. Given the data set for the African Americans, the transition probability matrix is expressed by:
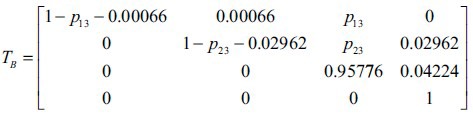
Here, we use an average value of rates over 2006–2009 in Table 1 to obtain transition probabilities. For instance, p12= 0.00066 is calculated by the average of 65.7 × 105, 65.7 × 105, 67.4 × 105 and 66.6 × 105. However, we cannot directly obtain the transition probabilities p13 and p23 from the given data set since these values are hidden among data. Therefore, we estimate unknown parameters in a way that minimises the mean squared error (MSE) of the rate of persons living with both a diagnosis of HIV infection and an AIDS diagnosis, and deaths of persons with HIV/AIDS diagnosis over 2006 to 2008 as expressed in the following equation:
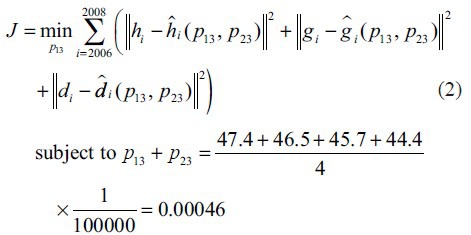
where hi is the reported rate of persons living with a diagnosis of HIV infection at the year of i; ĥi(p13, p23) (is the estimated rate of persons living with a diagnosis of HIV infection by the Markov chain with unknown parameter of p13 and p23 at the year of i; ĝi(p13, p23) is the reported rate of persons living with AIDS diagnosis at the year of i; is the estimated rate of persons living with AIDS diagnosis by the Markov chain with unknown parameter of p13 and p23 at the year of i; di is the reported rate of deaths of persons with either a diagnosis of HIV infection or AIDS diagnosis at the year of i; đi(p13, p23) and is the estimated rate of deaths of persons with either a diagnosis of HIV infection or AIDS diagnosis at the year of i by the Markov chain with unknown parameter of p13 and p23 at the year of i.
By solving Eqn 2, we are able to obtain the transition probabilities p13= 41 (per 100 000) and the transition matrix TB as follows:
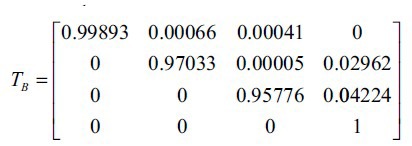
Applying an optimisation algorithm to estimate unknown parameters in a Markov chain is a novel and unique approach that is developed in this work, and is especially useful when available data is not enough or sometimes incomplete[19].
RESULTS
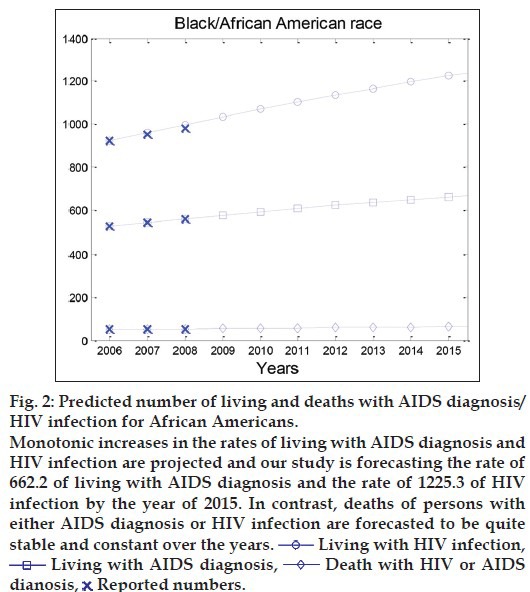
Based on the Markov model described above, the MATALB software has been used to numerically analyse HIV/AIDS progression. The predicted numbers of living with AIDS diagnosis and HIV infection, and deaths of persons with HIV/AIDS are displayed for the African Americans in fig. 2. Monotonic increases in the rates of living with AIDS diagnosis and HIV infection are projected and our study is forecasting the rate of 662.2 of living with AIDS diagnosis and the rate of 1225.3 of HIV infection by the year of 2015. In contrast, deaths of persons with either AIDS diagnosis or HIV infection are forecasted to be quite stable and constant over the years.
Fig. 2.
The same procedures to calculate the transition matrix TW of a Markov chain for the Caucasians are repeated.

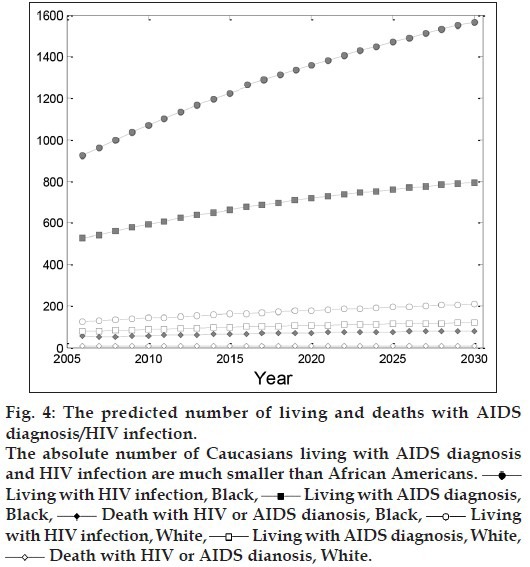
Fig. 3 shows a similarly increasing trend in Caucasians living with AIDS diagnosis and HIV infection compared with African Americans. However, the absolute number of Caucasians living with AIDS diagnosis and HIV infection are much smaller than African Americans. This discrepancy between both the races can be clearly seen in Table 2 and fig. 4, in which the numbers of living with AIDS diagnosis, HIV infection and deaths in both the races are illustrated simultaneously until 2030.
Fig. 3.
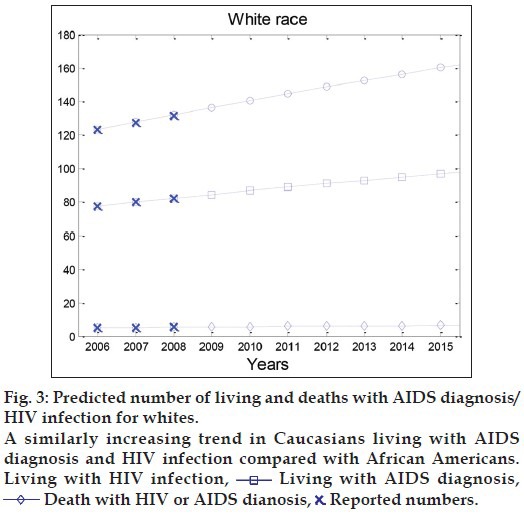
TABLE 2.
PREDICTED AIDS DIAGNOSIS/HIV INFECTION NUMBERS (IN THOUSANDS)
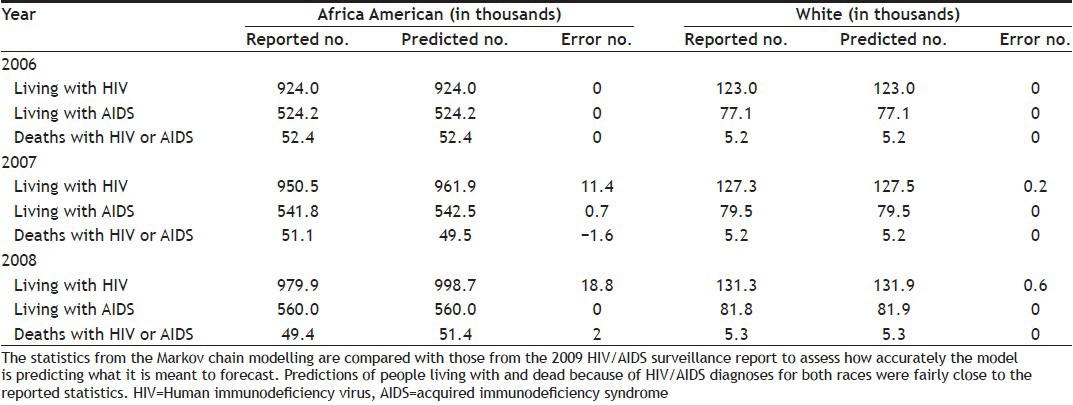
Fig. 4.
Model verification:
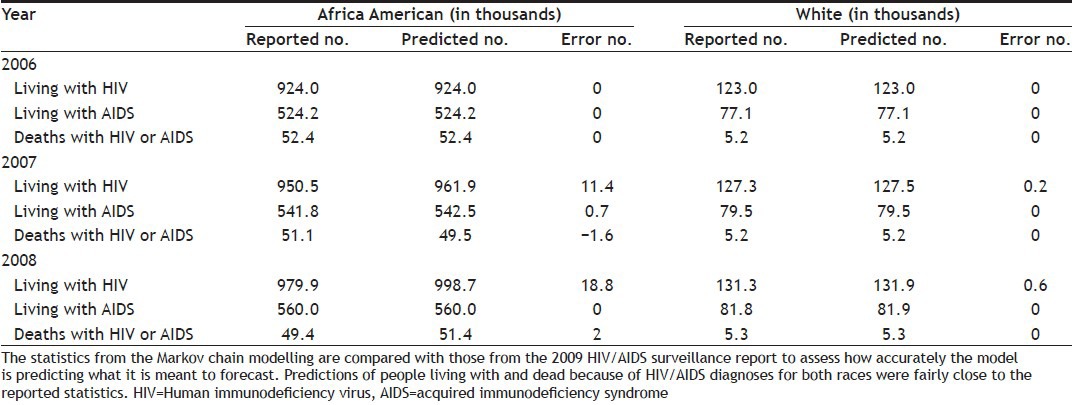
Computed results from the Markov chain modelling can be compared with reported statistics in Table 1 to assess how accurately the model is predicting what it is meant to forecast. Because data related to HIV/AIDS infections is handled confidentially across most parts of the country and infected people may hesitate to officially report it to CDC, the reported infection rates are only estimates. The CDC database comparisons of the calculated figures to the real figures will be done to verify the model. Table 3 shows the statistics from the Markov chain modelling against those from the 2009 HIV/AIDS surveillance report. Predictions of people living with and dead because of HIV/AIDS diagnoses for both races were fairly close to the reported statistics, as shown in Table 3.
TABLE 3.
MARKOV MODEL VERIFICATION WITH 2009 HIV SURVEILLANCE REPORT
DISCUSSION
The study shows that the number of HIV/AIDS diagnoses and HIV/AIDS related deaths each year is quite consistent, while the number of people living with HIV/AIDS among the infected population is increasing. The continuously increasing number of people who have HIV/AIDS reminds us of challenges associated with the disease, particularly the mortality and a lot of issues associated with perception of the disease, patient education and its long term management.
The trends suggest that the current HAART-based management of HIV/AIDS, which is evidence based, is relatively successful and effective. Furthermore, the consistently increasing rate of newly diagnosed HIV infections implies that current HIV preventive interventions or strategies are effective yet not sufficient for controlling the disease. To improve HIV/AIDS awareness, CDC and its partners have designated the day of February 7 as the National Black HIV/AIDS Awareness Day[4].
In our study, we also observed an increasing racial disparity related to HIV/AIDS. Government agencies such as CDC have implemented interventions or strategies that address racial disparities related to HIV/AIDS. Diverse HIV prevention efforts, such as national antiAIDS campaign, sexual health education, behavioural change intervention programmes targeted towards different vulnerable populations, have helped these affected people to get treatment[1]. CDC has also initiated relevant activities such as “Take Charge. Take the Test”, “Act Against AIDS Leadership Initiative (AAALI)”, and “Expanded Testing Initiative (ETI)”. Additionally, community-based organisations or foundations have also developed prevention interventions for the African American population, such as “Women Involved in Life Learning from Other Women (WILLOW)”, “Sister to sister” and “Nia”. Despite these efforts to address the issue, there remains a lack of persistent delivery, evaluation and improvement in the current programmes and interventions. Undoubtedly, all these efforts need collaboration of people working at the national level down and the community level for further success at the grass-roots level[1].
The cost of HIV-related lifetime health care in 1998 was $342 000, which increased to $600 000 in 2006, and the average monthly cost of $2100[20]. These findings imply that we should consider developing and implementing cost-effective HIV/AIDS care, preventive interventions or monitoring programmes to control the epidemic and ultimately reduce the HIV/AIDS-related health care costs. Structures interventions focusing on preventive measures such as increased use of condoms and avoiding multiple partners can be cost-effective in the long run. Similarly, the antiretroviral therapy (ART) for preventing mother-to-child HIV transmission amounts to a saving of US$ 34 per disability-adjusted life-year, whereas the full ART treatment to a pregnant woman living with HIV can be costly for the first few years but would lead to tremendous savings in the long run by saving the newly born from being orphans and HIV. Vulnerable populations such as sex workers or intravenous drug users might experience fear and tend to be mobile from one place to another since their visibility might lead to their arrest or violence from authorities. Cost-effective interventions focusing on collective action-based structural approaches such as providing peer led outreach, resolving crisis, providing temporary shelter and community mobilisation can help to reduce the risk taking behaviours of such individuals[2,21].
Healthcare providers treat diverse patients, including patients with language barriers, low health literacy with many unattended cultural needs. Hence approaches that portray the cultural sensitivity and cultural competency of the healthcare providers can fulfil the unmet needs of such patients[22]. Provision of culturally competent care can shift the focal point from providing cure for the ailment to the individual patient's needs. In case of African American patients, health care providers should bear certain behaviour traits of the patients in mind such as addressing patients formally, look in the patient's eyes while making conversation, explain the reasons for seeking personal information and try to gain trust of the patients during interactions with them[23].
The patient provider interactions in case of African American patients are more dominated by providers and patients tend to exchange less information with the providers. Hence, it is important for the providers to have engaging conversations with the patients by probing them for more information, making decisions collectively and by being a patient listener. Such actions on behalf of the providers can generate more respect in the minds of the patients and their perception of racism might reduce considerably[24]. Cultural competency interventions can be designed as per the needs of the patients, thereby provide practical solutions and make an impact in changing the health behaviour of the patients. Such interventions can help overcome stigma, mistrust and language barriers and can be more effective when conducted in partnerships with the community leaders, community workers and faith based organisations[25]. Considering the limited resource availability, we need interventions with the greatest potential to control the epidemic, for example the ones that mainly target the high risk populations and geographic areas[4]. Integrating cultural, social, contextual and interpersonal relationship factors that impact the sexual health in the African Americans can help researchers develop strategies that can be used in creating programmes on prevention, sexual health education and behavioural change. Research has shown that African Americans with HIV/AIDS tend to have lower ART adherence, mean CD4 T-cell count and immunological and virological responses to ART compared with Caucasians with HIV/AIDS[26,27,28,29,30,31,32]. Socioeconomic determinants such as lack of proper housing, health insurance, education and job security, environmental stressors, isolation by family and friends can impact the well-being of the HIV/AIDS patients and further increase the racial health disparity[22].
In order to design interventions that reduce disease transmission among the African Americans, it is important to understand their risk taking behaviour, not as being part of a single race but being part of individual subgroups like age, gender, sexuality and occupation[33]. HIV/AIDS in itself is associated with a lot of stigma among African Americans and particularly more for homosexual, bisexual or transgender patients, to an extent whereby such patients might experience isolation[33]. Ethnocentric research focusing on mental health issues (depression), cultural and religious factors can provide further insight about the stress inducing and risk taking behaviour among African Americans[34]. Providing public health educational messages about HIV prevention among the adolescent population and encouraging disease disclosure for protecting sexual partners and family members can reduce mistrust and improve awareness among the different subgroups in the African American community. If researchers have an understanding of the interpersonal relationships of the HIV positive patients with their family and friends, appropriate interventions can be designed that prevent social isolation. This can be done by focusing on social networks and incorporating religious and cultural resources such as churches and faith-based community organisations. Management of HIV/AIDS requires not only empowering patients but also training healthcare personnel. Providing equal opportunity and appropriate training to African American students in different healthcare sectors, recruiting and retaining African American faculty in academic institutions and including African American people in higher leadership roles can help to increase the rates of minority healthcare workers who can provide patient centred care to the African American patients[23]. HIV and sexual health training given to physicians can improve their ability to treat their patients and have open communication with them[33].
The primary concern to any government is to control widespread and severe epidemics such as HIV/AIDS. Health care needs for infected people can be planned well in advance in anticipation of new cases. The results from our study imply that ethnographic considerations should be incorporated in the HIV/AIDS prevention, management and programme evaluation interventions to optimise resource allocation. Our findings can be helpful and informative for the policy makers to make decisions and develop antiAIDS campaigns to determine proactive measures to counter the infection rate. Results from this study can be used for the supply chain logistic operations of antiretroviral drug manufacturers. The prospect of using this model to predict the prevalence of HIV/AIDS in other races looks promising.
However, there are still technical limitations in this work. The Markov model in this work is constructed based on the statistics in the HIV surveillance report only from 2006 to 2009 due to unavailability of previous years’ data. If more historical data were available, model estimation and prediction would be more precise. Since we model a HIV/AIDS progression with a homogeneous Markov model whose transition probabilities are stationary and constant, changes in transition behaviour and characteristics cannot be represented with this model.
Footnotes
Lee, et al.: Race Based Forecast of HIV/AIDS Progression
REFERENCES
- 1.Quinn TC. Global burden of the HIV pandemic. Lancet. 1996;348:99–106. doi: 10.1016/s0140-6736(96)01029-x. [DOI] [PubMed] [Google Scholar]
- 2.UN Joint Programme on HIV/AIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic. 2010. [Last accessed on 2013 May 27]. Available from: http://www.refworld.org/docid/4cfca9c62.html .
- 3.United States Centers for Disease Control and Prevention. HIV Surveillance Report, Volume 21. Diagnoses of HIV infection and AIDS in the United States and dependent areas. 2009. [Last accessed on 2013 May 27]. Available from: http://www.cdc.gov/hiv/pdf/statistics_2009_HIV_Surveillance_Report_vol_21.pdf .
- 4.United States Centers for Disease Control and Prevention. Fact sheets 2011: HIV among African Americans. 2011. [Last accessed on 2013 May 27]. Available from: http://www.cdc.gov/nchhstp/newsroom/docs/FastFacts.AA.FINAL508COMP.pdf .
- 5.Ross SM. Wiley Series in probability and statistics. 2nd ed. Hoboken, NJ: John Wiley and Sons Inc; 1995. Stochastic Processes. [Google Scholar]
- 6.Kuo HS, Chang HJ, Chou P, Teng L, Chen TH. A Markov chain model to assess the efficacy of screening for non-insulin dependent diabetes mellitus (NIDDM) Int J Epidemiol. 1999;28:233–40. doi: 10.1093/ije/28.2.233. [DOI] [PubMed] [Google Scholar]
- 7.Davies R, Johnson D, Farrow S. Planning patient care with a Markov model. Opl Res Q. 1975;26:599–607. [Google Scholar]
- 8.Kay R. A Markov model for analyzing cancer markers and disease states in survival studies. Biometrics. 1986;42:855–65. [PubMed] [Google Scholar]
- 9.Pérez-Ocón R, Ruiz-Castro JE, Gámiz-Pérez ML. Non-homogeneous Markov models in the analysis of survival after breast cancer. J R Stat Soc Ser C Appl Stat. 2001;50:111–24. [Google Scholar]
- 10.Jackson CH, Sharples LD. Hidden Markov models for the onset and progression of bronchiolitis obliterans syndrome in lung transplant recipients. Stat Med. 2002;21:113–28. doi: 10.1002/sim.886. [DOI] [PubMed] [Google Scholar]
- 11.Commenges D, Joly P, Letenneur L, Dartiques JF. Incidence and mortality of Alzheimer's disease or dementia using an illness-death model. Stat Med. 2004;23:199–210. doi: 10.1002/sim.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bature RS, Obiniyi AA, Absalom EE, Sule OO. Markov chain simulation of HIV/AIDS movement pattern. Int J Comput Sci. 2010;8:156–67. [Google Scholar]
- 13.Sweeting MJ, Farewell VT, De Angelis D. Multi-state Markov models for disease progression in the presence of informative examinationtimes: An application to hepatitis C. Stat Med. 2010;29:1161–74. doi: 10.1002/sim.3812. [DOI] [PubMed] [Google Scholar]
- 14.Debanne SM, Bielefeld RA, Cauthen GM, Daniel TM, Rowland DY. Multivariate Markovian modeling of tuberculosis: Forecast for the United States. Emerg Infect Dis. 2000;6:148–57. doi: 10.3201/eid0602.000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longini IM, Jr, Clark WS, Byers RH, Ward JW, Darrow WW, Lemp GF, et al. Statistical analysis of the stages of HIV infection using a Markov model. Stat Med. 1989;8:831–43. doi: 10.1002/sim.4780080708. [DOI] [PubMed] [Google Scholar]
- 16.Bailey NT. Prediction and validation in the public health modeling of HIV/AIDS. Stat Med. 1994;13:1933–43. doi: 10.1002/sim.4780131906. [DOI] [PubMed] [Google Scholar]
- 17.Schinaia G. Modeling the HIV/AIDS epidemic via survivor functions. Eur J Epidemiol. 2000;16:573–9. doi: 10.1023/a:1007663607280. [DOI] [PubMed] [Google Scholar]
- 18.Tan WY, Tang SC. A general Markov model of the HIV epidemic in populations involving both sexual contact and IV drug use. Math Comput Model. 1994;19:83–132. [Google Scholar]
- 19.Gentleman RC, Lawless JF, Lindsey JC, Yan P. Multi-state Markov models for analyzing incomplete disease history data with illustrations for HIV disease. Stat Med. 1994;13:805–21. doi: 10.1002/sim.4780130803. [DOI] [PubMed] [Google Scholar]
- 20.Schackman BR, Gebo KA, Walensky RP, Losina E, Muccio T, Sax PE, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44:990–7. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 21.Harper PR, Shahani AK. A decision support system for the care of HIV and AIDS patients in India. Eur J Oper Res. 2003;147:187–97. [Google Scholar]
- 22.American College of physician. Racial and ethnic disparities in health care, Updated. 2010. [Last accessed on 2013 May 27]. Available from: http://www.acponline.org/advocacy/current_policy_papers/assets/racial_disparities.pdf .
- 23.Cichocki M. Culturally Sensitive HIV Care-Be Aware of Cultural Differences. 2008. [Last accessed on 2013 May 27]. Available from: http://aids.about.com/cs/doctors/a/culture.htm .
- 24.Beach MC, Saha S, Korthuis PT, Sharp V, Cohn J, Wilson IB, et al. Patient-provider communication differs for black compared to white HIV-infected patients. AIDS Behav. 2011;15:805–11. doi: 10.1007/s10461-009-9664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaegi L. Nurses vs. HIV/AIDS disparities: Creating culturally competent interventions. Minority Nurse. 2007. [Last accessed on 2013 May 27]. Available from: http://www.minoritynurse.com/article/nurses-vs-hivaids-disparities-creating-culturally-competent-interventions .
- 26.Gifford AL, Bormann JE, Shively MJ, Wright BC, Richman DD, Bozzette SA. Predictors of self-reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. J Acquir Immune Defic Syndr. 2000;23:386–95. doi: 10.1097/00126334-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 27.Kleeberger CA, Phair JP, Strathdee SA, Detels R, Kingsley L, Jacobson LP. Determinants of heterogeneous adherence to HIV-antiretroviral therapies in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2001;26:82–92. doi: 10.1097/00126334-200101010-00012. [DOI] [PubMed] [Google Scholar]
- 28.Hartzell JD, Spooner K, Howard R, Wegner S, Wortmann G. Race and mental health diagnosis are risk factors for highly active antiretroviral therapy failure in a military cohort despite equal access to care. J Acquir Immune Defic Syndr. 2007;44:411–6. doi: 10.1097/QAI.0b013e31802f83a6. [DOI] [PubMed] [Google Scholar]
- 29.Golin CE, Liu H, Hays RD, Miller LG, Beck CK, Ickovics J, et al. A prospective study of predictors of adherence to combination antiretroviral medication. J Gen Intern Med. 2002;17:756–65. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith CJ, Sabin CA, Youle MS, Kinloch-de Loes S, Lampe FC, Madge S, et al. Factors influencing increases in CD4 cell counts of HIV-positive persons receiving long-term highly active antiretroviral therapy. J Infect Dis. 2004;190:1860–8. doi: 10.1086/425075. [DOI] [PubMed] [Google Scholar]
- 31.Kleeberger CA, Buechner J, Palella F, Detels R, Riddler S, Godfrey R, et al. Changes in adherence to highly active antiretroviral therapy medications in the Multicenter AIDS Cohort Study. AIDS. 2004;18:683–8. doi: 10.1097/00002030-200403050-00013. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Margolick JB, Conover CS, Badri S, Riddler SA, Witt MD, et al. Interruption and discontinuation of highly active antiretroviral therapy in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2005;38:320–8. [PubMed] [Google Scholar]
- 33.Wyatt GE, Williams JK, Myers HF. African-American Sexuality and HIV/AIDS: Recommendations for Future Research. J Natl Med Assoc. 2008;100:44. doi: 10.1016/s0027-9684(15)31173-1. [DOI] [PubMed] [Google Scholar]
- 34.Israelski DM, Prentiss DE, Lubega S, Balmas G, Garcia P, Muhammad M, et al. Psychiatric Co-morbidity in vulnerable populations receiving primary care for HIV/AIDS. AIDS Care. 2007;19:220–5. doi: 10.1080/09540120600774230. [DOI] [PubMed] [Google Scholar]


