Figure 1.
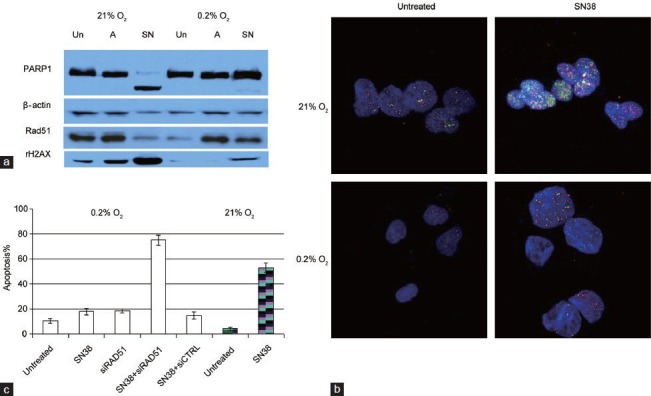
(a) Western blots comparing PARP1 cleavage (apoptosis marker) and levels of Rad51 and rH2AX (marker for double strand DNA breaks) in untreated (Un), ABT888-treated (A) or SN38-treated (SN) PC3 cells grown under 21% or 0.2% oxygen for 4 days. SN38 is the active metabolite of irinotecan. Compared to drug-treated PC3 cells under normoxia, less DNA damage and apoptosis were observed in treated PC3 cells under hypoxia. (b) Immunofluorescence of Rad51 (red) and γ-H2AX (green) foci in untreated or SN38-treated (0.1 μmol l–1 for 4 h) PC3 cells grown under normoxia (21% O2) or chronic hypoxia (0.2% O2 for 72 h). Increased Rad51 nuclear foci formation was observed soon after PC3 cells were challenged with SN38. Compared to SN38-treated cells under normoxia, there was less DNA damage under hypoxia. (c) Blocking Rad51 upregulation with siRNA-resensitized PC3 cells to SN38. Percentage of apoptosis was detected by flow cytometry with propidium iodide and annexin V. PC3 cells grown in 0.2% O2 were transfected with Rad51 siRNA or scrambled siRNA control (siCTRL); inhibiting Rad51 with siRad51 reversed the resistance to SN38 under hypoxic culture. Untreated and SN38-treated PC3 cells grown in 21% O2(hatched column) and 0.2% O2 were shown for comparison.
