Abstract
This study sought to assess the prognostic significance of the degree of extranodal extension (ENE) and several other risk factors in pathological ENE penile carcinoma. We analyzed prospectively collected data on a consecutive series of 31 chemotherapy-naive patients with proven ENE who underwent therapeutic regional lymphadenectomy. Postoperative external radiotherapy was then performed. We studied the extent of ENE utilizing a novel grading system and correlated patient grades with their outcome measures. ENE was graded as 1 - if the capsule of the lymph node (LN) was ruptured less than one-third of its circumference or 2 - if the capsule was disrupted more than one-third of its circumference or the entire LN was disrupted. We estimated overall survival (OS) using the Kaplan-Meier method. Multivariate analysis was performed according to the Cox proportional hazards model using factors that were identified as statistically significant in univariate analysis. The incidence rate of ENE was 51.8% in patients with pathological node-positive carcinoma of the penis. The median OS and 5-year survival were 18 months (95% confidence interval (CI), 14.4–21.6) and 23%, respectively. Prognostic variables on univariate analysis were ENE grade 2, ≥3 LNs with ENE, maximal LN ≥ 35 mm, ≥5 positive LNs and pelvic LN involvement. On multivariate analysis, only ENE grade 2 remained associated with decreased OS (hazard ratio (HR): 6.50). In conclusion, patients with ENE have a poor outcome, and ENE grade 2 is an independent predictive factor of poor OS in patients with pathological ENE penile carcinoma.
Keywords: extranodal extension, neoplasm metastasis, penile neoplasms, penis, prognosis
INTRODUCTION
Extranodal extension (ENE) of tumor cells beyond the capsule of the lymph nodes (LNs) is widely regarded as a prognostic factor in many solid tumors, such as breast cancer,1,2 cervical carcinoma,3 head and neck carcinoma,4 and penile squamous cell carcinoma (PSCC).5
The association of ENE with poorer outcomes in PSCC was first recognized in the 1980s, and several subsequent studies confirmed this association.6,7 However, previous studies on this subject have several limitations, including the following: the numbers of patients assessed were relatively small owing to the low incidence of PSCC, the definition of ENE that was used was not always reported, and there is no consensus on exactly what degree of ENE is most critical in terms of prognosis. The presence or absence of ENE has been predominantly reported as ‘yes’ or ‘no’, with no description or quantitation. In addition, the 5-year survival rate differed among these studies with a range from 0% to 42%, and we assume that the extent of ENE may be one reason for this variation.5,6,8
The purpose of this paper is to analyze the prognostic significance of the degree of ENE, by utilizing a novel grading system, in a series of 31 men with PSCC who underwent standard locoregional treatment and then being diagnosed ENE. At the same time, we correlated several other risk factors with patient outcomes. The data were collected prospectively.
PATIENTS AND METHODS
Patient selection and data collection
A prospective search was performed in the Fudan University Shanghai Cancer records for patients with PSCC who had documented evidence of at least one ENE-positive LN. We identified 61 consecutive patients with PSCC in whom LN metastases (LNMs) were surgically resected between January 2001 and January 2010. Patients who received neoadjuvant therapy were excluded. Of the 61 patients, 31 were diagnosed with pathological ENE and were included in the next analysis. All of the patients underwent standard bilateral inguinal lymphadenectomy (inguinal lymph node dissection (ILND)) and pelvic lymphadenectomy. Surgery was performed by four experienced staff urologists.
Information on age, maximum preoperative diameter of LNs, pathological tumor stage, histopathological grade, number of positive inguinal LNs, bilateral inguinal LN involvement or not, number of LNs with ENE, degree of ENE, bilateral inguinal LNs with ENE, ENE density and status of pelvic LNs was retrieved from the medical records. With the exception of age, diameter of LNs, ENE density and number of metastatic inguinal LNs, other factors were coded as categorical variables. ENE density was defined as the ratio of the number of ENE LNs to the total number of positive LNs.
Surgery and adjuvant radiotherapy indications
Penile tumors were treated surgically. Generally, T1 and T2 tumors smaller than 2 cm were treated with penis preserving methods, whereas partial amputation was performed for larger T2, T3 and T4 tumors. In several men, the primary tumor was surgically removed at the referring hospital, and these patients were referred for LN treatment.
All PSCC patients underwent bilateral ILND in the prophylactic or therapeutic setting at our center. Ipsilateral pelvic lymphadenectomy and subsequent adjuvant external radiotherapy were performed when histopathological examination of the inguinal dissected specimen showed ENE or when two or more inguinal LNs were involved. The borders of the ilioinguinal lymphadenectomy were previously described in detail.9 The radiotherapy dose was usually 50 Gy in 25 fractions of 2 Gy and 5 fractions per week.10
We excluded patients from analysis who had undergone neoadjuvant chemotherapy or previous groin surgery. Informed consent was obtained from all patients, and the protocol was approved by the institutional ethics committee.
Histopathology and ENE measurement
Penile tumors were staged according to the 2009 American Joint Committee on Cancer (AJCC) Tumor, Node, Metastasis (TNM) system, and histopathological grade (moderate or poorly differentiated) was also recorded. LNs were dissected manually and completely embedded in paraffin for serial sectioning. Histopathological findings were described in a standardized format. ENE was defined as extension of the tumor through the LN capsule into the perinodal fibrous-adipose tissue or squamous cell carcinoma in soft tissue with no evidence of LN architecture.5,11,12,13 At first, we attempted to measure the maximum linear distance from the external capsule border to the farthest extent of the tumor or the tumoral reaction, with the aid of a microscope. However, we found that when the capsule was broken more than one-third of its circumference or if the entire LN was disrupted, the specimen was deemed as being immeasurable. This phenomenon was also noted in previous reports, and the specimen was excluded from analysis. Here, nodal metastases were graded for the highest degree of ENE using the following simple novel grading system: grade 1 - the capsule of LN was ruptured less than one-third of its circumference, and grade 2 - the capsule was disrupted more than one-third of its circumference or the entire LN was disrupted. If a patient had two or more LNs with ENE, he was graded according to the LN with the highest stage. All of the measurements were assessed by two experienced genitourinary pathologists. Any discrepancies were resolved by jointly reviewing the slides.
Follow-up and statistical analysis
Patient follow-up was scheduled according to a standard protocol. Patients were seen postoperatively at 2-month intervals during the first 2 years, at 3-month intervals in year 3 and at 6-month intervals thereafter. The follow-up consisted of physical examination with ultrasound and computerized tomography as indicated. For this study, follow-up data until February 2013 were used. Clinical follow-up information was obtained from detailed clinician databases. This study's primary end point was median survival time. For statistical analysis, the surgery date was considered to be the start of the survival time. Overall survival (OS) ended either when a patient died due to any cause or at the date of the last known follow-up.
Categorical variables were analyzed using contingency tests (Fisher's exact test and chi-square test). Owing to the small sample size, all of the continuous data are dichotomized according to the median of each factor. All of the survival times and proportions were determined based on Kaplan-Meier estimates. Logrank tests were used to compare survival intervals upon covariates’ effects. Multivariate survival analysis was performed according to the Cox proportional hazards model of factors found to be statistically significant using univariate analysis with two-sided test (P < 0.05 considered to be statistically significant). All statistical analyses were performed using Statistical Package for Social Sciences software version 19.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient characteristics
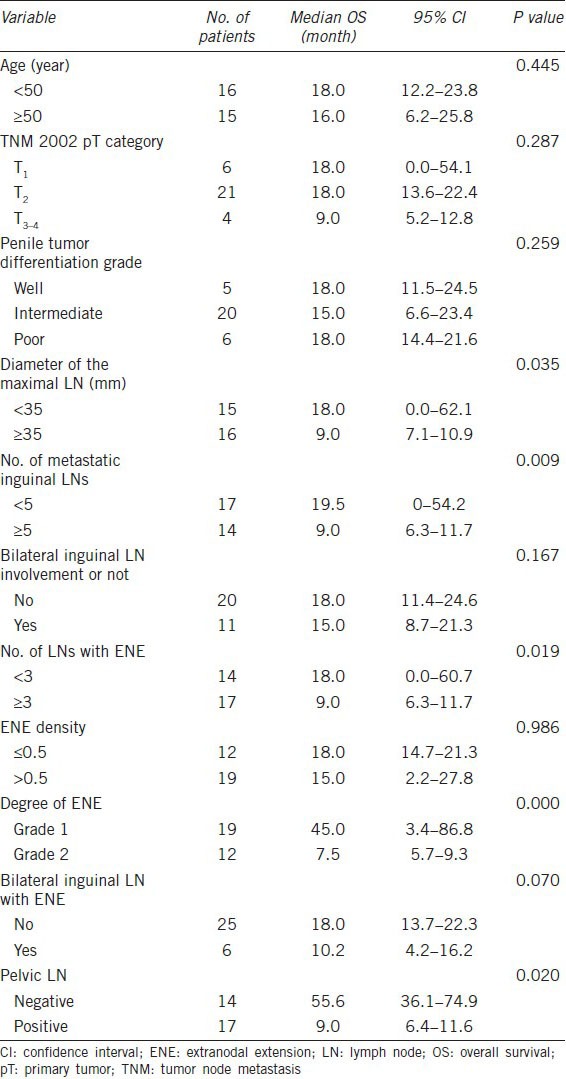
The incidence of ENE was 51.8% in patients with pathological node-positive penile carcinoma. Table 1 summarizes the clinicopathological characteristics of the 31 men, who had a median age of 49 years (range 38–84). A median count of nine LNs (range 2–28) was found and examined in each unilateral inguinal specimen, a median of 4 LNs (range, 1–19 LN) were tumor positive, a median of 3 LNs (range, 1–5 LN) were ENE and the median ENE density was 0.6 (range, 0.25–1). There were 19 ENE grade 1 cases and 12 ENE grade 2 cases. All of the patients had at least one mass in the groin, with a median diameter of 35 mm (range 11–60) for the largest LN according to the preoperative computed tomography or magnetic resonance imaging scan. Bilateral inguinal LN involvement occurred in 11 (35.5%) patients, six (19.4%) patients had bilateral inguinal ENE and 17 (54.8%) patients were diagnosed with pelvic LNM.
Table 1.
Clinicopathological characteristics and univariate analysis of variables associated with overall survival
Overall survival
The median follow-up was 18 months (range 3–93). A total of 23 men (74.2%) died after a median time of 14 months (range 2.5–55.5). Eight patients were alive at the deadline time with a median follow-up of 60.3 months (range 36–93). The median survival time and 5-year survival rate in the entire cohort were 18 months (95% confidence interval (CI), 14.4–21.6) and 23%, respectively.
We noted no survival differences among patients of different ages (P = 0.445, Table 1), tumor stages (P = 0.287), histopathological grades (P = 0.259) or ENE densities (P = 0.986) or number of groins with LNM (P = 0.167).
Patients with ENE grade 2 had a significantly decreased median survival time (7.5 vs 45 months, P = 0.000, Figure 1). Other variables, such as diameter of the maximal LN, number of positive inguinal LNs, number of LNs with ENE and pelvic LN involvement, were also prognostic factors in the univariate analysis (Table 1).
Figure 1.
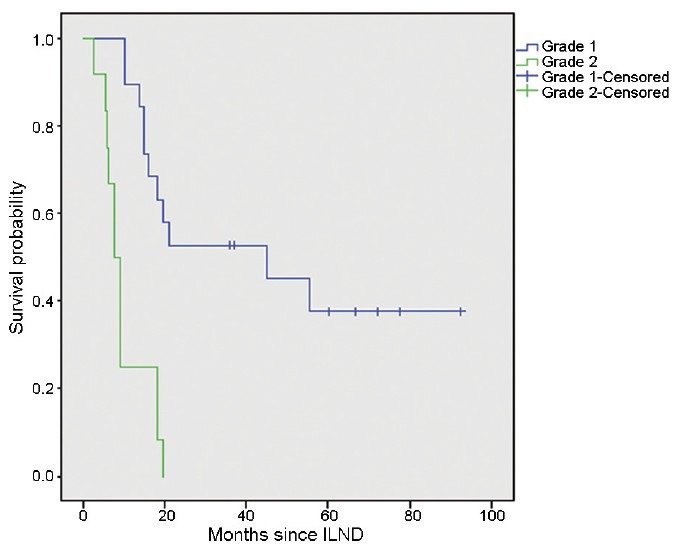
Overall survival of patients with ENE grade 1 or grade 2 (P = 0.000). Values indicate number of patients at risk. ENE: extranodal extension; ILND: inguinal lymph node dissection.
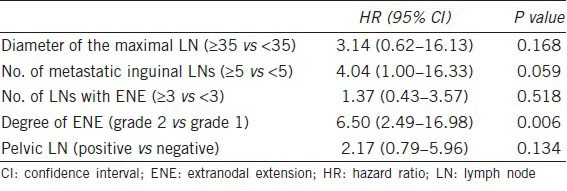
On multivariate analysis, only ENE grade 2 remained significantly associated with OS (hazard ratio (HR) 6.50; 95% CI, 2.49–16.98, Table 2). The HR of 6.50 indicates a more than six-fold relative risk of death due to penile carcinoma in men with ENE grade 2 vs men with ENE grade 1.
Table 2.
Multivariate analysis of overall survival
DISCUSSION
Our study of prognostic factors in ENE penile carcinoma reveals that OS is significantly affected by the degree of ENE. The incidence of ENE is a well-known adverse prognostic factor for recurrence, metastasis and survival in many solid cancers.1,2,3,4,14 In 1987, the association of ENE with poorer outcomes in PSCC was first suggested.7 Since that time, owing to the low incidence of PSCC, only a few studies have described this phenomenon.5,6,8 Pandey et al.6 and Lont et al.10 affirmed the significance of ENE and found that the 5-year survival rate was approximately 0%–15%. In 2009, a revised TNM staging system for penile cancer was published with a major change in the N categories, which changed ENE of regional LNM to N3 disease.8 In 2010, an Amsterdam-based group analyzed prospectively collected data from a series of 156 chemotherapy-naive patients diagnosed with LN involvement who underwent ILND.5 They found that men with ENE had a significantly shorter 5-year cancer specific survival than men without ENE (42% vs 80%); and when using multivariate analysis, ENE remained associated with decreased cancer-specific survival (HR 2.37).
Previous studies also reached the same conclusion, that is, patients with ENE were reported to have a worse prognosis. However, the 5-year survival rate varied considerably, with a range from 0% to 42%, in those studies, and it is unclear whether a larger extent of ENE further worsens the prognosis. Therefore, we initially attempted to analyze the survival information about ENE and preliminarily explore whether the degree of ENE is a prognostic factor for patients with ENE. We initially tried to measure the maximum linear distance from the external capsule border to the farthest extent of the tumor or the tumoral reaction. However, when the outer limit of the capsule was perforated more than one-third of its circumference, the specimen was deemed to be immeasurable. We also noted that many LNs were entirely disrupted by the tumor. It was therefore impossible to measure the distance of ENE in these cases. This phenomenon was also observed in some previous studies in which, in order to determine the optimal clinical target volume, margins around the gross nodal tumor volume in head-and-neck cancer were assessed by microscopically measuring the tumor extension beyond the cervical LN capsules. The specimens were excluded from analysis.11,12,13 Accordingly, we developed a novel grading system for the degree of ENE according to whether the capsule was destroyed around more or less than one-third of its circumference. To the best of our knowledge, the present study is the first to grade the extent of ENE in PSCC. The advantage of this system is its simplicity, and all LNs can be measured, in contrast to the previous method.
In our cohort, all of the men were diagnosed with LNM in the past 10 years, no one received neoadjuvant therapy, and the incidence of ENE was 51.8%, which is equivalent to that found in previous studies. The median survival time was 18 months (95% CI, 14.4–21.6), and the 5-year survival rate was 23%, which is lower than that reported by the Amsterdam-based group but higher than that reported in studies before 2007. In exploring the possible prognostic factors for ENE patients, we found that only the degree of ENE (grade 2 vs grade 1) is an independent predictive variable using multivariate analysis (the HR value is 6.50 and the median OS is shortened to 7.5 months from 45 months). To our knowledge, no study has been performed that predicts prognostic factors for PSCC with ENE, and only a few similar articles examined the degree of ENE when other tumors were found. Lewis et al.4 studied the correlation between outcomes and the degree of extracapsular extension in oropharyngeal squamous cell carcinoma, and they found that ENE grades did not correlate with nodal size and that ENE with no residual nodal tissue was associated with poorer survival times (P < 0.01), in an attempt to stratify different risk groups within a larger group of patients with extracapsular extension of tumors from primary laryngeal or hypopharyngeal squamous cell carcinoma. deCarvalho15 prospectively analyzed 170 consecutive cases and found that when the tumor was confined to the LN or showed only microscopic invasion beyond the capsule, there were no significant differences in risk rates for treatment failure. The presence of macroscopic penetration of the LN capsule by a tumor, however, increased the risk of recurrence by 3.5 times compared with patients in whom the tumor was confined to the LN. Macroscopic extracapsular spread as the major prognostic factor for recurrent disease in the neck has also been reported by Ferlito et al.12
It is easy for a pathologist to assess the degree of ENE, and the median survival time for men with grade 2 ENE is only 7.5 months despite treatment with adjuvant therapy. We suspect that men with grade 2 ENE or a tumor invading the surrounding soft tissue (even when the LNs present without a complete capsule), may have a more aggressive carcinoma, whose amount of postoperative circulating tumor cells and local positive margin rate is higher. Despite receiving standard adjuvant therapy, these patients usually have local recurrence and distant metastasis early, and their OS time is shorter. Understanding the level of ENE may help urologists communicate better with patients and prepare a prophylactic systemic treatment to improve the patient's outcome and quality of life. Additionally, the patient's family can also carry out the psychological preparation for each outcome.
Several previous studies suggested that the number of metastatic inguinal nodes affects survival in patients with node-positive penile cancer, and a significantly better outcome is seen in patients with fewer involved nodes. Patients with pelvic LN involvement are uniformly considered to have a poor prognosis with 0% to 20% 5-year survival.5,6 In this study, the cutoff to determine the poor prognosis group for OS on univariate analysis was 5 or greater metastatic inguinal nodes and pelvic LN involvement. Other factors such as the diameter of the maximal LN (more than 35 mm) and the number of LNs with ENE (≥ three) were shown to be associated with prognosis in univariate analysis. However, these features failed to be independent factors on multivariate analysis. An explanation for this outcome is that most patients with five or greater metastatic inguinal nodes or pelvic LN involvement also have grade 2 ENE, which is a stronger prognosticator. Another factor explaining this result may be the relatively small sample size because of the low prevalence of PSCC.16
In our study, tumor differentiation was not an independent factor in univariate analysis. Although some publications report that patients with well differentiated penile carcinoma have a higher survival rate than those with moderately and poorly differentiated carcinoma, most of the studies have found that tumor differentiation grade is not an indicator for poor prognosis for 5-year survival.17,18 Human papillomavirus and several molecular markers have been associated with the outcome of PSCC patients in some previous studies. Two studies found that the presence of LNMs is higher for patients with human papillomavirus DNA-negative; however, the P values are not significant.18,19 P53, Ki-67, E-cadherin, and epidermal growth factor receptor (EGFR) are suggested molecular prognostic markers in PSCC by some studies.16,20,21,22 Di Lorenzo et al.21 found that expression of p-EGFR was strongly associated with increased risk of recurrence and shorter OS for patients with N0–1 disease. However, it is unclear whether ENE correlates with human papillomavirus or other molecular markers, and further research is required.
How can we improve the outcome in men who have penile carcinoma with ENE? First, neoadjuvant or adjuvant systemic treatment is probably needed in those patients. In cases of head and neck carcinoma, adjuvant treatment has evolved from radiotherapy to chemoradiation in patients with ENE.23 This approach has resulted in improved locoregional control and consequently, improved survival, although at the cost of a substantial increase in adverse effects. Second, the potential may exist for EGFR inhibition with monoclonal antibodies (panitumumab and cetuximab), and angiogenesis is also a promising target. In one retrospective study, all 13 patients with advanced PSCC expressed EGFR, with 77% exhibiting a 3 + level of expression and received EGFR-targeted therapies. These patients showed a median time-to-progression of 3.2 months and a median OS of 9.8 months.24 In a retrospective report of six chemorefractory patients following at least two prior regimens treated with sunitinib or sorafenib, one patient achieved a partial response and four patients developed stable disease.25
Our study had several limitations: (i) the study population was collected from a single tertiary center in China. Therefore, the characteristics of our patients may be different from those of their counterparts at other centers. (ii) The small sample size restricted the number of variables during multivariate analysis. (iii) We did not investigate the relationship between molecular markers and ENE. Nevertheless, our study is an important first step in developing a novel grading system for PSCC with ENE and identifies ENE grades as a significant prognostic factor for PSCC.
CONCLUSIONS
In conclusion, our study confirmed that PSCC with ENE has a worse outcome than men with no ENE. We are the first group to measure the degree of ENE and to propose a grading system for PSCC. Multivariate analysis of several histopathological factors for OS revealed that only the ENE degree was an independent prognostic factor, despite these patients having received surgery with postoperative radiotherapy. Therefore, systemic treatment is probably needed to improve the outcome.
AUTHOR CONTRIBUTIONS
JYW and YZ designed the study, collected, analyzed and interpreted the clinical data, wrote the manuscript and revised the manuscript. HLZ and SXT reviewed pathological slides and revised the manuscript. DWY supervised the project and revised the manuscript. XJQ, SLZ and BD collected partial patients’ clinical data and performed patient follow-ups. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by the Grants for International Cooperation and the Exchange of Science and Technology Commission of Shanghai Municipality (No. 12410709300) and a grant from the Guide Project of Science and Technology Commission of Shanghai Municipality (No. 124119a7300).
REFERENCES
- 1.Gorgulu S, Can MF, Yagci G, Sahin M, Tufan T. Extracapsular extension is associated with increased ratio of metastatic to examined lymph nodes in axillary node-positive breast cancer. Clin Breast Cancer. 2007;7:796–800. doi: 10.3816/CBC.2007.n.042. [DOI] [PubMed] [Google Scholar]
- 2.Neri A, Marrelli D, Roviello F, De Stefano A, Guarnieri A, et al. Prognostic significance of positive axillary lymph node matastases and extracapsular extension in T1 to T3 breast cancer. Ann Surg Oncol. 2005;12:246–53. doi: 10.1245/ASO.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 3.Horn LC, Hentschel B, Galle D, Bilek K. Extracapsular extension of pelvic lymph node metastases is of prognostic value in carcinoma of the cervix uteri. Gynecol Oncol. 2008;108:63–7. doi: 10.1016/j.ygyno.2007.08.086. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JS, Jr, Carpenter DH, Thorstad WL, Zhang Q, Haughey BH. Extracapsular extension is a poor predictor of disease recurrence in surgically treated oropharyngeal squamous cell carcinoma. Mod Pathol. 2011;24:1413–20. doi: 10.1038/modpathol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graafland NM, van Boven HH, van Werkhoven E, Moonen LM, Horenblas S. Prognostic significance of extranodal extension in patients with pathological node positive penile carcinoma. J Urol. 2010;184:1347–53. doi: 10.1016/j.juro.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Pandey D, Mahajan V, Kannan RR. Prognostic factors in node-positive carcinoma of the penis. J Surg Oncol. 2006;93:133–8. doi: 10.1002/jso.20414. [DOI] [PubMed] [Google Scholar]
- 7.Srinivas V, Morse MJ, Herr HW, Sogani PC, Whitmore WF. Penile cancer: relation of extent of nodal metastasis to survival. J Urol. 1987;137:880–2. doi: 10.1016/s0022-5347(17)44281-9. [DOI] [PubMed] [Google Scholar]
- 8.In: Edge SB, Byrd DR, Carducci MA, editors. 7th ed. New York: Springer; 2009. AJCC Cancer Staging Manual. [Google Scholar]
- 9.Zhu Y, Zhang SL, Ye DW, Yao XD, Dai B, et al. Prospectively packaged ilioinguinal lymphadenectomy for penile cancer: the disseminative pattern of lymph node metastasis. J Urol. 2009;181:2103–8. doi: 10.1016/j.juro.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 10.Lont AP, Kroon BK, Gallee MP, van Tinteren H, Moonen LM, et al. Pelvic lymph node dissection for penile carcinoma: extent of inguinal lymph node involvement as an indicator for pelvic lymph node involvement and survival. J Urol. 2007;177:947–52. doi: 10.1016/j.juro.2006.10.060. [DOI] [PubMed] [Google Scholar]
- 11.Coatesworth AP, MacLennan K. Squamous cell carcinoma of the upper aerodigestive tract: The prevalence of microscopic extracapsular spread and soft tissue deposits in the clinically N0 neck. Head Neck. 2002;24:258–61. doi: 10.1002/hed.10020. [DOI] [PubMed] [Google Scholar]
- 12.Ferlito A, Rinaldo A, Devaney KO, MacLennan K, Myers JN, et al. Prognostic significance of microscopic and macroscopic extracapsular spread from metastatic tumor in the cervical lymph nodes. Oral Oncology. 2002;38:747–51. doi: 10.1016/s1368-8375(02)00052-0. [DOI] [PubMed] [Google Scholar]
- 13.Apisarnthanarax S, Elliott DD, El-Naggar AK, Asper JA, Blanco A, et al. Determining optimal clinical target volume margins in head-and-neck cancer based on microscopic extracapsular extension of metastatic neck nodes. Int J Radiat Oncol Biol Phys. 2006;64:678–83. doi: 10.1016/j.ijrobp.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Heide J, Krull A, Berger J. Extracapsular spread of nodal metastasis as a prognostic factor in rectal cancer. Int J Radiat Oncol Biol Phys. 2004;58:773–8. doi: 10.1016/S0360-3016(03)01616-X. [DOI] [PubMed] [Google Scholar]
- 15.Brasilino de Carvalho M. Quantitative analysis of the extent of extracapsular invasion and its prognostic significance: a prospective study of 170 cases of carcinoma of the larynx and hypopharynx. Head Neck. 1998;20:16–21. doi: 10.1002/(sici)1097-0347(199801)20:1<16::aid-hed3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Sonpavde G, Pagliaro LC, Buonerba C, Dorff TB, Lee RJ, et al. Penile cancer: current therapy and future directions. Ann Oncol. 2013;24:1179–89. doi: 10.1093/annonc/mds635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ornellas AA, Kinchin EW, Nóbrega BL, Wisnescky A, Koifman N, et al. Surgical treatment of invasive squamous cell carcinoma of the penis: brazilian national cancer institute long-term experience. J Surg Oncol. 2008;97:487–95. doi: 10.1002/jso.20980. [DOI] [PubMed] [Google Scholar]
- 18.Lont AP, Kroon BK, Horenblas S, Gallee MP, Berkhof J, et al. Presence of high-risk human papillomavirus DNA in penile carcinoma predicts favorable outcome in survival. Int J Cancer. 2006;119:1078–81. doi: 10.1002/ijc.21961. [DOI] [PubMed] [Google Scholar]
- 19.Bezerra AL, Lopes A, Santiago GH, Ribeiro KC, Latorre MR, et al. Human papillomavirus as a prognostic factor in carcinoma of the penis: analysis of 82 patients treated with amputation and bilateral lymphadenectomy. Cancer. 2001;91:2315–21. [PubMed] [Google Scholar]
- 20.Zhu Y, Zhou XY, Yao XD, Dai B, Ye DW. The prognostic significance of p53, Ki-67, epithelial cadherin and matrix metalloproteinase-9 in penile squamous cell carcinoma treated with surgery. BJU Int. 2007;100:204–8. doi: 10.1111/j.1464-410X.2007.06908.x. [DOI] [PubMed] [Google Scholar]
- 21.Di Lorenzo G, Perdonà S, Buonerba C, Sonpavde G, Gigantino V, et al. Cytosolic phosphorylated EGFR is predictive of recurrence in early stage penile cancer patients: a retropective study. J Transl Med. 2013;11:161–7. doi: 10.1186/1479-5876-11-161. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Lorenzo G, Buonerba C, Gaudioso G, Gigantino V, Quarto G, et al. EGFR mutational status in penile cancer. Expert Opin Ther Targets. 2013;17:501–5. doi: 10.1517/14728222.2013.783571. [DOI] [PubMed] [Google Scholar]
- 23.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–52. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 24.Carthon B, Pettaway C, Pagliaro L. Epidermal growth factor receptor (EGFR) targeted therapy in advanced metastatic squamous cell carcinoma (AMSCC) of the penis: updates and molecular analyses. J Clin Oncol. 2010;28:e15022. [Google Scholar]
- 25.Zhu Y, Li H, Yao XD, Zhang SL, Zhang HL, et al. Feasibility and activity of sorafenib and sunitinib in advanced penile cancer: a preliminary report. Urol Int. 2010;85:334–40. doi: 10.1159/000315432. [DOI] [PubMed] [Google Scholar]


