Abstract
In western populations, prostate volume (PV) has been proven to be one of the strongest predictors of detecting prostate cancer (PCa) in biopsies. We performed this study in a biopsy cohort, to evaluate associations among the prostate volume, prostate-specific antigen (PSA) and PCa detection in the Chinese population. Between the years, 2007–13, 1486 men underwent prostate biopsy at Huashan Hospital, Fudan University, Shanghai, China. The study population was divided into two groups for analysis according to total PSA (tPSA) range (4 ng ml−1 < tPSA ≤20 ng ml−1 and tPSA > 20 ng ml−1). PV, age, tPSA, digital rectal examination (DRE) and transrectal ultrasound (TRUS) results were also included in the analysis. Although the positive biopsy rates decreased in both tPSA range groups, the downtrend was more pronounced in the 4 ng ml−1 < tPSA ≤20 ng ml−1 group; therefore, we focused on 853 men in this group with increasing PV. In multivariate logistic regression analysis, only DRE was found to be associated with PCa in four PV groups (P < 0.05) and tPSA did not show a good predictive ability when PV exceeded 50 ml (P > 0.05). Further, it may suggest that with increasing PV, the cancer detection rate decreased in men with different tPSA, DRE and TRUS nodule statuses (all P values for trends were <0.001). Our study indicates that in tPSA ranging from 4 to 20 ng ml−1, the use of PV ranges of 0–35 ml, 35–50 ml and > 50 ml might be taken into consideration for the biopsy decision-making in the Chinese population.
Keywords: China, prostate cancer, prostate-specific antigen, prostate volume
INTRODUCTION
Prostate cancer (PCa) is the second most common cancer and one of the leading causes of death among the men population worldwide.1 The incidence of PCa in China is relatively low compared to developed countries; however, it has been progressively rising over the past decades.2
Prostate-specific antigen (PSA) is the most widely used biomarker for PCa screening and prostate biopsy decision making. Over the past decades, PSA elevation has become the most common indication for prostate biopsy.
Several factors may cause the level of PSA to rise, e.g., enlargement of prostate volume (PV), inflammation of the prostate, extra secretion from tumor cells, and so on. Thus, a slight increase of PSA does not definitely indicate PCa. Several PSA derivatives, such as the ratio of free to total PSA (% fPSA), PSA density (PSAD) and PSA velocity (PSAV) were introduced to increase the accuracy for predicting PCa in different populations.3,4,5,6 As an independent risk factor, PV has been one of the strongest predictors of PCa detection in transrectal ultrasound (TRUS)-guided prostate biopsy.6,7,8,9 However, most studies were focused on the western populations within the PSA gray zone, which ranged from 2.0 to 10.0 ng ml−1, except for one study that was based on a relatively small Chinese population with PSA values which ranged from 10.0 to 50.0 ng ml−1.9 The PSA gray zone was initially determined in western populations, so it may not be appropriate for the Chinese population due to the difference of PCa incidence between different ethnic groups. Studies have shown that the PCa rate in Chinese men population with PSA ranging from 4.0 to 10.0 ng ml−1 was around 20%,10,11,12 which is comparatively lower than the data reported in western population.7,13 Thus, the PSA gray zone in the Chinese men population should have a wider range. Regarding the limited data of associations between PV, PSA and PCa detection in the Chinese men population, we performed this study based on the biopsies of Chinese population to evaluate the ability of PV to predict PCa in men within different PSA ranges.
MATERIALS AND METHODS
Study population and sample collection
Total of 1486 men who underwent prostate biopsy from January 2007 to March 2013 at Huashan Hospital, Fudan University, Shanghai, China were included as the candidates of the current study. Patients were excluded from this study if the total PSA (tPSA) or transrectal ultrasound results were missing. As one of the leading tertiary health institutes in China, patients from all over the country come to Huashan Hospital for medical services. The characteristics of tertiary health institutes in China have been described in our previous study.12
All the candidates underwent 10-core ultrasound-guided transperineal prostate biopsy test based on a fixed template. The indications for prostate biopsy at our institute were: (1) tPSA > 4.0 ng ml−1 ; (2) tPSA < 4.0 ng ml−1, with suspicious fPSA/tPSA < 0.16 or PSA density (PSAD = tPSA/PV, PV (ml) = height (cm) × length (cm) × width (cm) ×0.52) > 0.15; (3) positive findings from digital rectal exam (DRE), with any level of tPSA; (4) positive findings from imaging techniques such as transrectal ultrasound (TRUS) and magnetic resonance imaging (MRI), with any level of tPSA. All specimens were diagnosed by the Pathology Department of Huashan Hospital. Blood samples were collected prior to biopsy examination and were measured by the Department of Clinical Laboratory for tPSA and fPSA. Results of DRE, TRUS (having nodule or not) and other clinical information were collected. Written informed consent was obtained from the patients for their participation in the study. This study was approved by the Institutional Review Board of Huashan Hospital, Fudan University, Shanghai, China.
Statistical analysis
The study population was divided into two groups according to tPSA range (4 ng ml−1 < tPSA ≤20 ng ml−1 and tPSA > 20 ng ml−1). The Chi-square trend test was used to evaluate the trend of positive rates in different groups. The men in the 4 ng ml−1 < tPSA ≤20 ng ml−1 group were stratified by median PV (< 50 ml and ≥50 ml). Logistic regressions of univariate and multivariate were used to evaluate the prediction ability of each variable (age, PSA, DRE and TRUS results) for predicting PCa in different groups. The positive rates of PCa among men in the 4 ng ml−1 < tPSA ≤20 ng ml−1 group with different tPSA ranges, DRE statuses and TRUS statuses stratified by PV quartiles were calculated. A two-sided testing with the Pvalue of 0.05 was used in the current study. Statistical analyses were performed using SPSS 19.0 (Statistical Product and Service Solutions, IBM Corporation, Armonk, NY, USA).
RESULTS
Within this cohort, 1445 men with tPSA measuring > 4 ng ml−1 and having complete PV data were included in the study.
The positive rates of the prostate biopsies among men with different tPSA ranges stratified by PV are shown in Figure 1 and Figure 2. Of the 853 men in the 4 ng ml−1 < tPSA ≤20 ng ml−1 group, the positive rate of PCa decreased remarkably from 66.7% to 9.0% with the increase of PV (Ptrend = 1.82 × 10−26) (Figure 1). Of the 592 men in the tPSA > 20 ng ml−1 group, the positive rate of PCa decreased from 90.9% to 55.2% with increasing PV (Ptrend = 2.70 × 10−7) (Figure 2). Although the positive biopsy rates decreased in both tPSA range groups, the rate decreased to less than 10% in the 4 ng ml−1 < tPSA ≤20 ng ml−1 group with PV larger than 60 ml whereas the rate remained relatively high (55.2%) with PV larger than 70 ml in the tPSA > 20 ng ml−1 group. Data from our cohort also showed that the positive biopsy rate of the patients with 20 ng ml−1 < tPSA ≤50 ng ml−1 was 56.6% (data not shown), which was much higher than that of patients with 4 ng ml−1 < tPSA ≤20 ng ml−1 group (24.2%). Therefore, patients with tPSA > 20 ng ml−1, should definitely undergo a prostate biopsy test due to higher positive biopsy rates found. Thus our following analysis mainly focused on 853 men in the 4 ng ml−1 < tPSA ≤20 ng ml−1 group.
Figure 1.
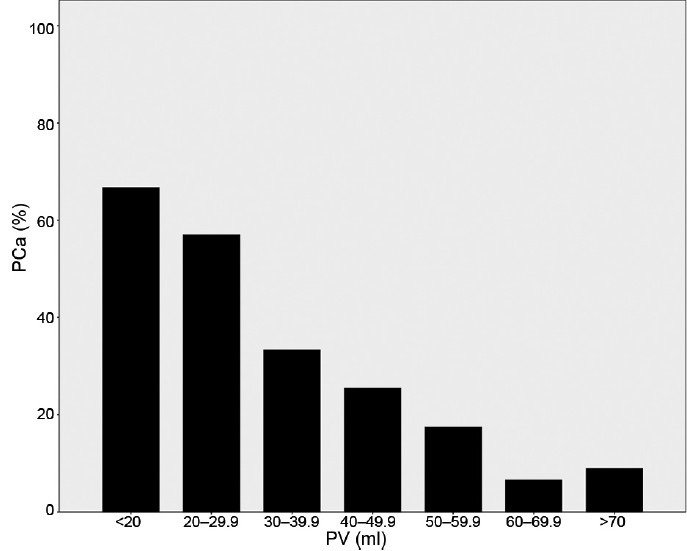
The positive rate of prostate cancer among men (n = 853) with tPSA from 4 to 20 ng ml−1. The proportion of prostate cancer decreases with increasing prostate volume (P-trend = 1.82 × 10−26). PCa: prostate cancer; PV: prostate volume.
Figure 2.

The positive rate of prostate cancer among men (n = 592) with tPSA >20 ng ml−1 stratified by prostate volume. The proportion of prostate cancer slightly decreases with increasing prostate volume (P-trend = 2.70 × 10−7). PCa: prostate cancer; PV: prostate volume.
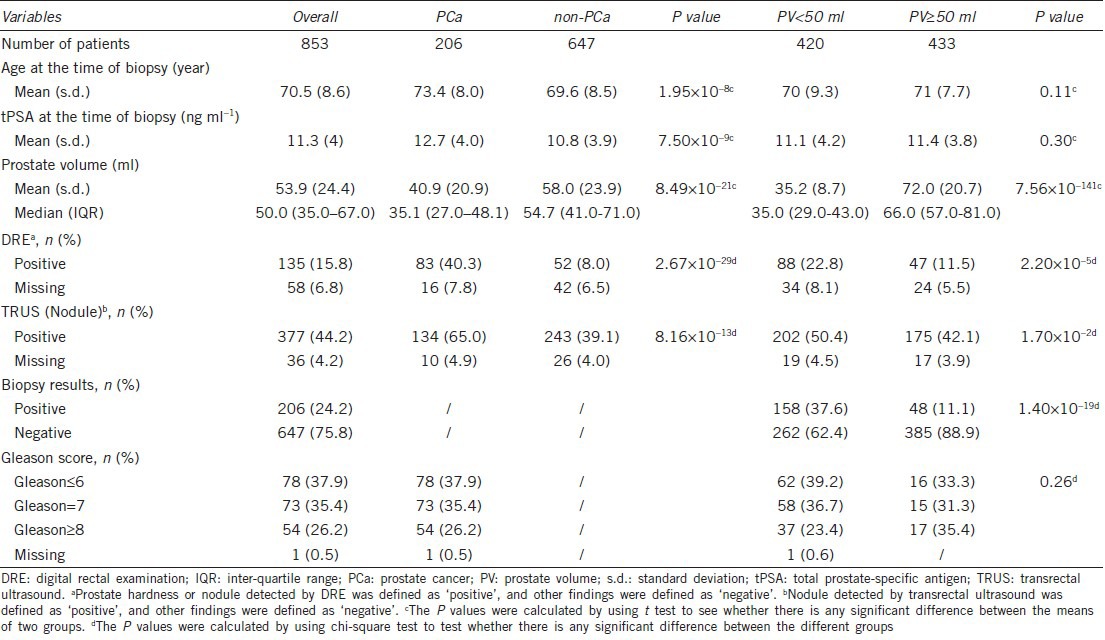
The demographic characteristics of the study population with 4 ng ml−1 < tPSA ≤20 ng ml−1 group are shown in Table 1. Of the 853 patients, 206 (24.2%) were diagnosed with PCa. The mean age of the study group was 70.5 years and the mean tPSA was 11.3 ng ml−1. The mean and median PVs of the cohort were 53.9 ml and 50.0 ml, respectively. The mean age and tPSA were statistically higher in men diagnosed with PCa compared with the men without PCa (P < 0.001) whereas no significant difference of these two variables was observed between different PV groups (P > 0.05). The mean PV in the non-PCa group compared with the PCa group was remarkably higher. The positive rates of DRE and TRUS were statistically higher in the PCa group and PV < 50 ml group than the non-PCa and PV ≥50 ml group (P < 0.05).
Table 1.
Characteristics of the study cohort with 4 ng ml−1 <tPSA≤20 ng ml−1
We first stratified the patients by PV quartiles into four groups, and then performed univariate analysis to test the association between PCa and each variable (age, tPSA, DRE, TRUS) (Table 2). Age and DRE were found to be associated with PCa in four groups (P < 0.05). PSA was associated with PCa when PV was less than 67 ml (P < 0.05); however, when PV exceeded 67 ml, the increase of tPSA may not be substantiate enough in detecting the risk of PCa in this specified tPSA range (P > 0.05). Further, the multivariate logistic regression analysis was performed to test the association between PCa and each variable (age, tPSA, DRE, TRUS) (Table 2). Only DRE was found to be associated with PCa in the four groups (P < 0.05). Age was found to be associated with PCa in the PV < 35 ml group (P < 0.05). When PV exceeded 50 ml, tPSA did not show a good predictive ability (P > 0.05).
Table 2.
Univariate and multivariate analyses of variables at the time of prostate biopsy in predicting the risk of prostate cancer

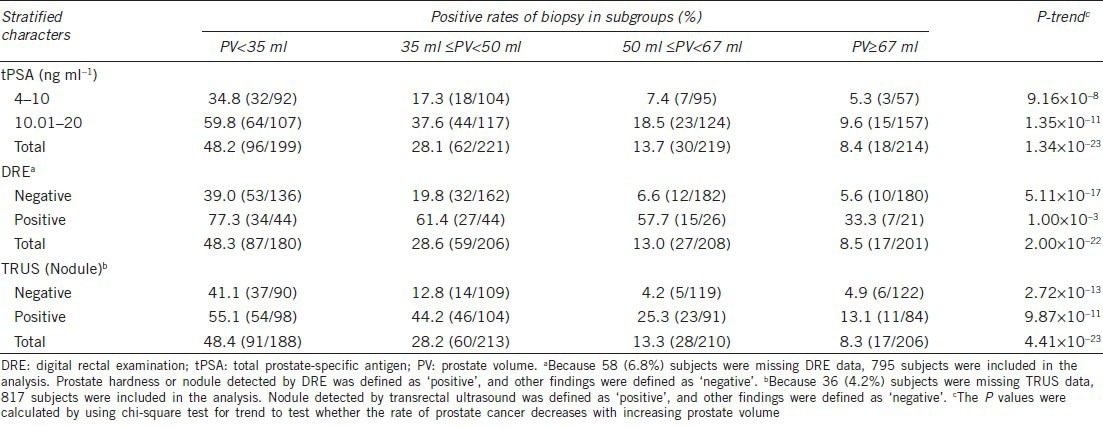
The positive rates of PCa among men with different PSA ranges, DRE and TRUS nodule statuses stratified by PV quartiles are shown in Table 3. The rates of PCa in men with PV < 35 ml, 35 ml ≤ PV < 50 ml, 50 ml ≤ PV < 67 ml and PV ≥ 67 ml in the 4 ng ml−1 < tPSA ≤10 ng ml−1 groups were 34.8%, 17.3%, 7.4% and 5.3%, respectively, whereas the rates of PCa in men with PV < 35 ml, 35 ml ≤PV < 50 ml, 50 ml ≤PV < 67 ml and PV ≥67 ml in the 10 ng ml−1 < tPSA ≤20 ng ml−1 groups were 59.8%, 37.6%, 18.5% and 9.6%, respectively. The above mentioned results suggest that with increasing PV, the cancer detection risk decreased in both the entire population and the stratified population that underwent 10-core TRUS-guided biopsy. Such a trend could also be observed in men with different DRE and TRUS nodule statuses (all P for trends were < 0.001).
Table 3.
Positive rates of biopsy among men with different PSA ranges, DRE and TRUS Nodule statuses, stratified by PV quartiles
DISCUSSION
To our knowledge, this study was the first comprehensive study to examine the association between PV, PSA and PCa detection risk based on a relatively large Chinese prostate biopsy cohort. We found that the risk of detecting PCa decreased with increasing PV in TRUS-guided prostate biopsy.
The goal of PCa screening is to identify curable disease while minimizing unnecessary biopsies. It was generally assumed that the detection risk of PCa by the biopsy test was linearly associated with elevated levels of PSA. In western and Chinese populations, due to the relatively low detection rate of cancer in men with low levels of PSA, the need to improve PCa detection strategies remains.8,10,11 In this study, we found that the PCa detection rate was 24.2% in men with 4 ng ml−1 < tPSA ≤20 ng ml−1 and higher than 55.2% in men with tPSA > 20 ng ml−1. Although the positive rates showed significant trends, the positive rate of biopsy was high enough to undertake prostate biopsy for men with tPSA > 20 ng ml−1. The positive rate of PCa in men with tPSA levels of 4 to 20 ng ml−1 was comparable with the PCa detection rate in western populations with 2 ng ml−1 < tPSA ≤ 10 ng ml−1,8,14 and was similar to a recent report from Guangdong Province, China and a report from Beijing, China.11,15 Data from the current study also showed that the positive biopsy rate of patients with the level of 20 ng ml−1 < tPSA ≤50 ng ml−1 was 56.6% (data not shown), which was much higher than that (24.2%) of patients with the level of 4 ng ml−1 < tPSA ≤20 ng ml−1. Thus we focused on patients with the level of 4 ng ml−1 < tPSA ≤20 ng ml−1 rather than the level of 10 ng ml−1 ≤ tPSA ≤50 ng ml−1 mentioned in another study in the Chinese population.9 Consequently, our conclusions drawn from this study can be applied for counseling patients with the level of 4 ng ml−1 < tPSA ≤20 ng ml−1 regarding PCa risk in the Chinese population.
The reason why less PV indicates a higher risk of detecting PCa in biopsy remains a discussion. Chen et al.16 examined 180 prostatectomy specimens and found small-volume cancers (0.5 ml or less) were twice as frequent in prostates larger than 50 ml. These small-volume tumors comprised 33% of cancers in prostates larger than 50 ml, 16% in prostates less than 30 ml, and 14% in prostates 30 to 50 ml. Thus, they reached the conclusion that the observed lower cancer detection rate in large prostates was due to the higher proportion of low volume cancers in these prostates. Patients with a large prostate are more likely to be biopsied because of benign prostatic hyperplasia (BPH) which causes PSA elevation rather than clinically significant PCa. Furthermore, due to the small volume of tumors in large prostates, the increasing number of cores may lead to a disproportionately increased detection rate of PCa in these prostates. Another study including histological examination of 875 radical prostatectomy specimens concluded that BPH is a strong contender that contributes to PSA elevation in patients with PSA below 9 ng ml−1.5 These results were consistent with our data that men with larger PV had a lower rate of detection of PCa in the 4 ng ml−1 < tPSA ≤20 ng ml−1 cohort. However, reports from Duke University held some different opinions; one of their studies showed that taking more biopsy cores improves the diagnostic performance for identifying unilateral PCa.17 In another study, including the retrospective analysis of 859 patients undergoing radical prostatectomy, they found that men with a prostate > 40 g (the author used gram in the original study and they assume 1 g = 1 ml) have higher tumor volumes and are more likely to have unilateral PCa than those with a smaller prostate. In addition, in prostates of more than 40 g, increasing the number of biopsy cores increased the diagnostic performance for detecting cancer laterality which could help to select patients for focal therapy.18 Further, some researchers consider that the inadequate sampling in TRUS-guided sextant biopsy in men with larger prostates would explain the decreased PCa detection rate in large prostates, and hence more extensive biopsy should be chosen for the patients with large prostates so that the bias could be reduced.19,20 A study comparing the sextant and extended 11-core multisite biopsies found that a 33% increase of cancer detection was observed in extended biopsy. This suggested that the 11-core multisite directed biopsy enhanced cancer detection compared to conventional sextant biopsy.21 These studies suggest that increasing biopsy cores would give a better performance in patients with large prostates. Recently, a systematic review and meta-analysis reached the conclusion that a saturation prostate biopsy scheme would detect about an extra 5% PCa than an extended scheme for men with lower PSA levels and higher PV.22 Thus, we believe that both BPH and under sampling contribute to the observed result in our study that the increasing PV is associated with decreased PCa detection rate.
In our study, in the 4 ng ml−1 < tPSA ≤20 ng ml−1 cohort of 853 patients, we found significant differences in age, tPSA, PV, DRE and TRUS results between PCa and non-PCa groups. In terms of PV, the non-PCa group showed a much higher mean level than the PCa group. There were no statistical differences in age and tPSA between the PV < 50 ml and PV ≥50 ml groups, although there were statistical differences in DRE and TRUS results in the two PV groups. The similarity in age and tPSA between the two PV groups could lead to the equal chance of performing biopsy in patients with different PVs who had different risk of detecting PCa. Since all patients underwent extended biopsy with 10 cores in our institution, this procedure should have reduced the bias caused by under sampling to a certain extent. However, in our study the 10-core biopsy was indeed a relative limitation, as we could not evaluate the extent of the biopsy scheme to influence the biopsy results in this study.
Meanwhile, we performed univariate and multivariate analyses including four variables (age, PSA, DRE, TRUS) in four PV groups. We found that when the PV exceeded 50 ml, tPSA did not show a good predictive ability for PCa in the tPSA range from 4 to 20 ng ml−1. This indicates we may not rely on tPSA levels for biopsy decision when a patient has a prostate larger than 50 ml whose tPSA ranges from 4 to 20 ng ml−1. Furthermore, we examined that the PCa detection risk among men with different PSA ranges, DRE and TRUS nodule statuses in different PV groups. This provided a clear overall view that a man with larger PV had a lower rate of PCa detection in biopsy irrespective of the PSA range or were the statuses of DRE and TRUS existed. Overall, due to the relatively low positive biopsy rate, we consider our analysis of PV in Chinese patients with 4 ng ml−1 < tPSA ≤20 ng ml−1 cohort was of clinical value that would help to reduce some unnecessary biopsies.
Another limitation of this study is that it is a retrospective study and the data were obtained from a single health institute. Nevertheless, as patients from all over the country come to our department for medical services, the population in our study could still be representative of the entire Chinese population.
CONCLUSIONS
Our study indicates that in the tPSA range from 4 to 20 ng ml−1, the use of the PV ranges of 0–35 ml, 35–50 ml and > 50 ml might be taken into consideration for prostate biopsy decision-making in the Chinese population in the clinical practice.
AUTHOR CONTRIBUTIONS
YSW, RN, QD, JFX conceived and designed the study. YSW, HWJ performed the experiments. YSW, RN analyzed the data. PDB, YSW contributed materials and analysis tools. YSW, RN wrote the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was sponsored by National Natural Science Foundation of China (No. 81202001 and No. 81202269), Program for New Century Excellent Talents in University, China (NCET-13-0136), Shanghai Education Committee Scientific Research Innovation Project, China (No. 14ZZ010).
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Liu M, Wang JY, Zhang YG, Zhu SC, Lu ZH, et al. Detection of urological and male genital tumors diagnosed in Beijing hospital 1995-2004. Zhonghua Yi Xue Za Zhi. 2007;87:2423–5. [PubMed] [Google Scholar]
- 3.Gann PH, Ma J, Catalona WJ, Stampfer MJ. Strategies combining total and percent free prostate specific antigen for detecting prostate cancer: a prospective evaluation. J Urol. 2002;167:2427–34. [PubMed] [Google Scholar]
- 4.Lam JS, Cheung YK, Benson MC, Goluboff ET. Comparison of the predictive accuracy of serum prostate specific antigen levels and prostate specific antigen density in the detection of prostate cancer in hispanic-american and white men. J Urol. 2003;170:451–6. doi: 10.1097/01.ju.0000074707.49775.46. [DOI] [PubMed] [Google Scholar]
- 5.Stamey TA, Johnstone IM, McNeal JE, Lu AY, Yemoto CM, et al. Preoperative serum prostate specific antigen levels between 2 and 22 ng/ml. correlate poorly with post-radical prostatectomy cancer morphology: prostate specific antigen cure rates appear constant between 2 and 9 ng/ml. J Urol. 2002;167:103–11. [PubMed] [Google Scholar]
- 6.Colleselli D, Bektic J, Schaefer G, Frauscher F, Mitterberger M, et al. The influence of prostate volume on prostate cancer detection using a combined approach of contrast-enhanced ultrasonography-targeted and systematic grey-scale biopsy. BJU Int. 2007;100:1264–7. doi: 10.1111/j.1464-410X.2007.07174.x. [DOI] [PubMed] [Google Scholar]
- 7.Al-Azab R, Toi A, Lockwood G, Kulkarni GS, Fleshner N. Prostate volume is strongest predictor of cancer diagnosis at transrectal ultrasound-guided prostate biopsy with prostate-specific antigen values between 2.0 and 9.0 ng/mL. Urology. 2007;69:103–7. doi: 10.1016/j.urology.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 8.Bruno JJ, 2nd, Armenakas NA, Fracchia JA. Influence of prostate volume and percent free prostate specific antigen on prostate cancer detection in men with a total prostate specific antigen of 2.6 to 10.0 ng/ml. J Urol. 2007;177:1741–4. doi: 10.1016/j.juro.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 9.Tang P, Jin XL, Uhlman M, Lin YR, Deng XR, et al. Prostate volume as an independent predictor of prostate cancer in men with PSA of 10-50 ng ml -1. Asian J Androl. 2013;15:409–12. doi: 10.1038/aja.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng XY, Xie LP, Wang YY, Ding W, Yang K, et al. The use of prostate specific antigen (PSA) density in detecting prostate cancer in Chinese men with PSA levels of 4–10 ng/mL. J Cancer Res Clin Oncol. 2008;134:1207–10. doi: 10.1007/s00432-008-0400-8. [DOI] [PubMed] [Google Scholar]
- 11.Tang P, Du W, Xie K, Deng X, Fu J, et al. Transition zone PSA density improves the prostate cancer detection rate both in PSA 4.0-10.0 and 10.1-20.0 ng/ml in Chinese men. Urol Oncol. 2013;31:744–8. doi: 10.1016/j.urolonc.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Na R, Jiang H, Kim ST, Wu Y, Tong S, et al. Outcomes and trends of prostate biopsy for prostate cancer in Chinese men from 2003 to 2011. PLoS ONE. 2012;7:e49914. doi: 10.1371/journal.pone.0049914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuda T, Saika K. Comparison of time trends in prostate cancer incidence (1973-2002) in Asia, from cancer incidence in five continents, Vols IV-IX. Jpn J Clin Oncol. 2009;39:468–9. doi: 10.1093/jjco/hyp077. [DOI] [PubMed] [Google Scholar]
- 14.Vickers AJ, Sjoberg DD, Ankerst DP, Tangen CM, Goodman PJ, et al. The prostate cancer prevention trial risk calculator and the relationship between prostate-specific antigen and biopsy outcome. Cancer. 2013;119:3007–11. doi: 10.1002/cncr.28114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Na YQ. The detection rate of prostate cancer in different prostate-specific antigen (PSA) levels in Chinese men. Zhonghua Yi Xue Za Zhi. 2008;88:16–8. [PubMed] [Google Scholar]
- 16.Chen ME, Troncoso P, Johnston D, Tang K, Babaian RJ. Prostate cancer detection: relationship to prostate size. Urology. 1999;53:764–8. doi: 10.1016/s0090-4295(98)00574-3. [DOI] [PubMed] [Google Scholar]
- 17.Tsivian M, Kimura M, Sun L, Mouraviev V, Mayes JM, et al. Predicting unilateral prostate cancer on routine diagnostic biopsy: sextant vs extended. BJU Int. 2010;105:1089–92. doi: 10.1111/j.1464-410X.2009.08904.x. [DOI] [PubMed] [Google Scholar]
- 18.Tsivian M, Moreira DM, Sun L, Mouraviev V, Kimura M, et al. Biopsy accuracy in identifying unilateral prostate cancer depends on prostate weight. Urol Oncol. 2012;30:21–5. doi: 10.1016/j.urolonc.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Basillote JB, Armenakas NA, Hochberg DA, Fracchia JA. Influence of prostate volume in the detection of prostate cancer. Urology. 2003;61:167–71. doi: 10.1016/s0090-4295(02)02103-9. [DOI] [PubMed] [Google Scholar]
- 20.Karakiewicz PI, Baizet M, Aprikian AG, Trudel C, Aronson S, et al. Outcome of sextant biopsy according to gland volume. Urology. 1997;49:55–9. doi: 10.1016/S0090-4295(96)00360-3. [DOI] [PubMed] [Google Scholar]
- 21.Babaian RJ, Toi A, Kamoi K, Troncoso P, Sweet J, et al. A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J Urol. 2000;163:152–7. [PubMed] [Google Scholar]
- 22.Jiang X, Zhu S, Feng G, Zhang Z, Li C, et al. Is an initial saturation prostate biopsy scheme better than an extended scheme for detection of prostate cancer? A systematic review and meta-analysis. Eur Urol. 2013;63:1031–9. doi: 10.1016/j.eururo.2013.01.035. [DOI] [PubMed] [Google Scholar]


