Abstract
Objectives
Antibacterial primer and adhesive are promising to inhibit biofilms and caries. Since restorations in vivo are exposed to saliva, one concern is the attenuation of antibacterial activity due to salivary pellicles. The objective of this study was to investigate the effects of salivary pellicles on bonding agents containing a new monomer dimethylaminododecyl methacrylate (DMADDM) or nanoparticles of silver (NAg) against biofilms for the first time.
Methods
DMADDM and NAg were synthesized and incorporated into Scotchbond Multi-Purpose adhesive and primer. Specimens were either coated or not coated with salivary pellicles. A microcosm biofilm model was used with mixed saliva from ten donors. Two types of culture medium were used: an artificial saliva medium (McBain), and Brain Heart Infusion (BHI) medium without salivary proteins. Metabolic activity, colony-forming units (CFU), and lactic acid production of plaque microcosm biofilms were measured (n = 6).
Results
Bonding agents containing DMADDM and NAg greatly inhibited biofilm activities, even with salivary pellicles. When using BHI, the pre-coating of salivary pellicles on resin surfaces significantly decreased the antibacterial effect (p < 0.05). When using artificial saliva medium, pre-coating of salivary pellicles on resin did not decrease the antibacterial effect. These results suggest that artificial saliva yielded medium-derived pellicles on resin surfaces, which provided attenuating effects on biofilms similar to salivary pellicles. Compared with the commercial control, the DMADDM-containing bonding agent reduced biofilm CFU by about two orders of magnitude.
Significance
Novel DMADDM- and NAg-containing bonding agents substantially reduced biofilm growth even with salivary pellicle coating on surfaces, indicating a promising usage in saliva-rich environment. DMADDM and NAg may be useful in a wide range of primers, adhesives and other restoratives to achieve antibacterial and anti-caries capabilities.
Keywords: Antibacterial dental adhesive, Quaternary ammonium, Silver nanoparticles, Dental plaque microcosm biofilm, Salivary pellicle
1. Introduction
Composites are popular dental filling materials because of their esthetics and improved handling and load-bearing properties [1–6]. After bonded into a tooth cavity with an adhesive, the composite restoration is expected to perform oral functions durably [7–10]. However, nearly half of all restorations fail within 10 years, and replacing them accounts for 50–70% of all restorative dentistry [11,12]. One main problem is that composites tend to accumulate more biofilms than other restorative materials in vivo [13,14]. Furthermore, gap formation can be observed between the adhesive and the primed dentin, or between the adhesive and the hybrid layer [15,16]. Hence, microleakage can occur and biofilms at the restoration margins can penetrate into the bonded interface, producing acids and causing secondary caries, which is the main reason for restoration failure [17,18]. Therefore, antibacterial composites and adhesives are needed to combat biofilms and caries.
Novel polymers containing quaternary ammonium salts (QAS) were developed [19–25]. Monomers such as 12-methacryloyloxydodecylpyridinium bromide (MDPB) and other antibacterial monomers could copolymerize with dental resins to form antibacterial polymer matrices that can effectively reduce bacteria growth [19,20,23,25–28]. Adhesives bond the composite restoration to the tooth structure [29–35]. Hence antibacterial adhesives containing MDPB were developed to inhibit bacteria at the tooth-restoration margins [20,36]. In addition, a methacryloxylethyl cetyl dimethyl ammonium chloride (DMAE-CB) adhesive also inhibited biofilm growth [37]. These polymerizable cationic monomers covalently bonded within the polymer matrix and killed bacteria upon contact. In addition to QAS, silver particles were also used to provide antibacterial activity to resinous materials [38,39]. Nanoparticles of silver (NAg) were well dispersed in the resinous matrix to exert antibacterial activity for adhesives and composites [24,40,41].
The human oral cavity supports a diverse microbial consortium comprising of hundreds of bacterial species [42,43]. Saliva in the oral cavity can be absorbed onto dental restoration surfaces to provide anchor points for bacteria [44] and may block certain functional groups of material surfaces. Several publications suggested that proteins adsorbed from physiological fluids, such as saliva-derived protein films, are able to attenuate the antibacterial properties of the underlying surfaces significantly [45–47].
In our previous studies, antibacterial resins containing a quaternary ammonium dimethacrylate (QADM) were developed [24,28,40]. More recently, a new quaternary ammonium monomer, dimethylaminododecyl methacrylate (DMADDM), was synthesized, which showed much stronger antibacterial activity than the previously-used QADM in adhesives [48]. However, the effects of salivary pellicle covering the resin containing DMADDM on its antibacterial potency have not been reported.
Therefore, the objectives of this study were to develop antibacterial adhesive and primer containing DMADDM and NAg, and to investigate their effects on microcosm biofilm properties with or without human salivary pellicle coverage. Microcosm biofilms were inoculated with mixed saliva from ten human donors. Human saliva without bacteria provided the salivary pellicles. Two types of culture medium were tested: (1) BHI medium which contained no salivary proteins, to compare human salivary pellicle-covered resin surfaces with non-pellicle surfaces; and (2) McBain medium which contained proteins to mimic saliva, to compare human salivary pellicle-covered resin surfaces with medium-derived pellicle surfaces. The following hypotheses were investigated: (1) human salivary pellicle coating will significantly reduce the antibacterial efficacy of DMADDM and NAg containing adhesive with biofilms cultured in BHI medium; (2) human salivary pellicle coating will not decrease the antibacterial efficacy of DMADDM and NAg adhesive with biofilms cultured in McBain medium; (3) adhesives containing DMADDM and NAg will be strongly antibacterial even when the resin surface was covered with human salivary pellicles.
2. Materials and methods
2.1. Antibacterial adhesive system containing DMADDM
Scotchbond Multi-Purpose bonding system (3 M, St. Paul, MN) was used as the parent bonding system and referred as “SBMP”. According to the manufacturer, SBMP etchant contained 37% phosphoric acid. SBMP primer single bottle contained 35–45% 2-hydroxyethylmethacrylate (HEMA), 10–20% copolymer of acrylic and itaconic acids, and 40–50% water. SBMP adhesive contained 60–70% BisGMA and 30–40% HEMA.
DMADDM was a quaternary ammonium methacrylate, and was recently synthesized and incorporated into composites [48]. The synthesis used a modified Menschutkin reaction, where a tertiary amine group was reacted with an organo-halide [23,24]. A benefit of this reaction is that the products are generated at virtually quantitative amounts and require minimal purification. Ten mmol of 1-(dimethylamino)docecane (Sigma, St. Louis, MO) and 10 mM of 2-bromoethyl methacrylate (BEMA, Monomer-Polymer and Dajec Labs, Trevose, PA) were combined with 3 g of ethanol in a 20 mL scintillation vial. The vial was stirred at 70 ºC for 24 h. The solvent was then removed via evaporation, yielding DMADDM as a clear, colorless, and viscous liquid. DMADDM was mixed with SBMP primer at a DMADDM/(SBMP primer + DMADDM) mass fraction of 5%, following previous studies [36,48]. The same 5% mass fraction of DMADDM was incorporated into SBMP adhesive.
2.2. Antibacterial adhesive system containing NAg
Silver 2-ethylhexanoate powder (Strem, New Buryport, MA) was dissolved in 2-(tert-butylamino)ethyl methacrylate (TBAEMA, Sigma) at 0.08 g of silver salt per 1 g of TBAEMA [39]. TBAEMA was used because it improves the solubility by forming Ag N coordination bonds with Ag ions, thereby facilitating the Ag salt to dissolve in the resin solution. TBAEMA contains reactive methacrylate groups and therefore can be chemically incorporated into a dental resin upon photopolymerization [39,40]. This method produced NAg with a mean silver particle size of 2.7 nm, which were well dispersed in the cured resin matrix [39,40]. Ag was incorporated into SBMP primer at silver 2-ethylhexanoate/(primer + silver 2-ethylhexanoate) mass fraction of 0.1%, following a previous study [41]. The same 0.1% mass fraction of silver was incorporated in SBMP adhesive.
Therefore, three groups were tested: (1) SBMP primer “P”, SBMP adhesive “A” (termed P&A control); (2) SBMP primer + 5% DMADDM, SBMP adhesive + 5% DMADDM (termed P&A + DMADDM); (3) SBMP primer + 0.1% NAg, SBMP adhesive + 0.1% NAg (termed P&A + NAg).
2.3. Resin specimen preparation
The cover of a sterile 96-well plate was used for specimen preparation [37]. Ten μL of a primer was placed in the bottom of the dent of the cover. After drying with a stream of air, 10 μL of adhesive was applied and photo-polymerized for 20 s. Then, a composite (TPH, Caulk/Dentsply, Milford, DE) was placed and photo-cured for 1 min. This yielded a tri-layered primer/adhesive/composite disk of approximately 8 mm in diameter and 1 mm in thickness. The cured disks were immersed in water and agitated for 1 h to remove any uncured monomers, following previous studies [20,41]. This treatment ensured that the subsequent antibacterial property measurement was due to the antibacterial resin, and not due to a short-term burst release of uncured monomers. The disks were then sterilized with ethylene oxide (Anprolene AN 74i, Andersen, Haw River, NC).
2.4. Saliva collection for the dental plaque microcosm model and salivary pellicle
The dental plaque microcosm model was approved by the University of Maryland. Human saliva was shown to be ideal for growing plaque microcosm biofilms in vitro, with the advantage of maintaining much of the complexity and heterogeneity of the dental plaque in vivo [43]. Saliva was collected from ten healthy adult donors having natural dentition without active caries or periopathology, and without the use of antibiotics within the past 3 months [40]. Participants did not brush teeth for 24 h and abstained from food/drink intake for at least 2 h prior to donating saliva. An equal amount of saliva from each of the ten donors was mixed together. Half of the saliva was diluted in sterile glycerol to a concentration of 30%, and stored at −80 ºC to be used as biofilm inoculums [40]. The other half of the saliva was used for creating salivary pellicles on resin surfaces. The saliva was centrifuged for 15 min at 12,000 × g to remove debris and then annealed for 30 min at 60 ºC, to kill the bacteria but not denature the proteins. After filtration through a 0.45 μm cellulose acetate filter (Corning, New York, NY), the saliva with proteins but without bacteria was frozen at −80 ºC for usage in salivary pellicle formation on resin surfaces [45].
2.5. Saliva pellicle treatment and types of growth medium
The tri-layered primer/adhesive/composite disks were divided into two groups. Group 1 received salivary pellicle coatings; group 2 had no salivary pellicles. Saliva treatment was performed by immersing the cured specimens in 1 mL of filter-sterilized saliva for 2 h at 37 ºC; a previous study showed that this immersion resulted in salivary pellicles covering the specimen surface [45]. The specimens were then used for bacteria inoculation.
Two types of growth medium were used to culture the bacteria. The first was Brain Heart Infusion broth which contained no salivary proteins (BHI, Becton Dickinson, Sparks, MD), following previous studies [45–47]. The second was an artificial saliva named the McBain medium, with the purpose to mimic saliva and enable the maintenance of complex, stable salivary microcosms [40,41,49]. McBain medium contained mucin (type II, porcine, gastric) at a concentration of 2.5 g/L; bacteriological peptone, 2.0 g/L; tryptone, 2.0 g/L; yeast extract, 1.0 g/L; NaCl, 0.35 g/L, KCl, 0.2 g/L; CaCl2, 0.2 g/L; cysteine hydrochloride, 0.1 g/L; haemin, 0.001 g/L; vitamin K1, 0.0002 g/L, at pH 7 [43,49]. Mucin is similar to that in natural saliva and can cover the resin specimen surfaces similar to natural saliva. Mucin accounts for about 26% of salivary proteins in natural saliva. Bacteriological peptone, tryptone and yeast extract provided nutrition for bacterial growth.
2.6. Dental plaque microcosm biofilm culture
Each resin disk, with or without salivary pellicle, was placed into a well of 24-well plates, with the primer surface on the top. The saliva-glycerol stock was added, with 1:50 final dilution, to either the BHI or the McBain medium as inoculum. An inoculum of 1.5 mL was added to each well, and incubated in 5% CO2 at 37 ºC for 8 h. The disks were then transferred to new 24-well plates with fresh medium. After 16 h, the disks were transferred to new 24-well plates with fresh medium and incubated for 24 h. This constituted 2 days of incubation, which was shown to form plaque microcosm biofilms on resin disks [40,41].
2.7. Live/dead bacterial viability assay
Specimens with 2-day biofilms were washed with phosphate buffered saline (PBS). Then, the biofilms were stained using a live/dead BacLight bacterial kit (Molecular Probes, Eugene, OR). Live bacteria were stained with Syto 9 to produce a green fluorescence. Bacteria with compromised membranes were stained with propidium iodide to produce a red fluorescence. Six specimens were examined for each group with confocal laser scanning microscopy (CLSM 510, Carl Zeiss, Thornwood, NY) [40,41]. A magnification of 200 was used in taking the images. The green channel was with 488 nm excitation and 514 nm emission. The red channel was with 543 nm excitation and 570 nm emission.
2.8. MTT metabolic assay
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) is a colorimetric assay that measures the enzymatic reduction of MTT, a yellow tetrazole, to formazan [23,24]. Resin disk with 2-day biofilm was transferred into new 24-well plate with 1 mL of MTT dye (0.5 mg/mL MTT in PBS) in each well and incubated at 37 ºC in 5% CO2 for 1 h. During this process, metabolically active bacteria reduced the MTT to purple formazan. After 1 h, the biofilm specimens were transferred to a new 24-well plate. An aliquot of 1 mL of dimethyl sulfoxide (DMSO) was added to each well to solubilize the formazan crystals. After incubation in the dark for 20 min, 200 μL of the DMSO solution was transferred to a 96-well plate, and the absorbance at 540 nm (optical density OD540) was measured via a microplate reader (SpectraMax M5, Molecular Devices, Sunnvale, CA) [23,24].
2.9. Lactic acid production and colony-forming unit (CFU) counts
Disks with 2-day biofilms were rinsed in cysteine peptone water (CPW) to remove loose bacteria and then placed in a new 24-well plate. An aliquot of 1.5 mL of buffered peptone water (BPW) supplemented with 0.2% sucrose was added into each well. The relatively high buffer capacity of BPW prevented the pH from becoming significantly acidic, as a low pH would hinder bacterial acid production. Biofilm were incubated at 5% CO2 and 37 ºC for 3 h to allow bacteria to produce acid. After 3 h, the BPW solutions were stored for lactate analysis. Lactate concentrations in the BPW solutions were determined using an enzymatic (lactate dehydrogenase) method [40]. The microplate reader was used to measure the absorbance at 340 nm for the BPW solutions. Standard curves were prepared using a lactic acid standard (Supelco Analytical, Bellefonte, PA) [40,41].
Disks with biofilms were transferred into tubes with 2 mL CPW, and the biofilms were harvested by sonication and vortexing (Fisher, Pittsburgh, PA) [40,41]. Three types of agar plates were prepared. First, tryptic soy blood agar culture plates were used to determine total microorganisms [40,41]. Second, mitis salivarius agar (MSA) culture plates, containing 15% sucrose, were used to determine total streptococci [40,41,50]. This is because MSA contains selective agents crystal violet, potassium tellurite and trypan blue, which inhibit most gram-negative bacilli and most gram-positive bacteria except streptococci, thus enabling streptococci to grow [50]. Third, cariogenic mutans streptococci are known to be resistant to bacitracin, and this property is often used to isolate mutans streptococci from the highly heterogeneous oral microflora. Hence, MSA agar culture plates plus 0.2 units of bacitracin per mL was used to determine mutans streptococci [40,41,51]. The bacterial suspensions were serially diluted and spread onto agar plates for CFU analysis [40,41].
2.10. Statistical analysis
All data collected from this research were first checked for normal distribution with the Kolmogorov–Smirnov test and tested for homogeneity with the Levene’s test. For MTT metabolic assay and acid production study, inter-group differences were estimated by a statistical analysis of variance (ANOVA) for factorial models; individual groups were compared with Fisher’s protected least-significant difference test. For CFU, the values were first transformed by Log10 to normalize the data distribution and then subjected to ANOVA and Fisher’s protected least-significant difference test. Statistical analyses were performed by SPSS 13.0 software (SPSS Inc., Chicago, IL) at a significance level of p < 0.05.
3. Results
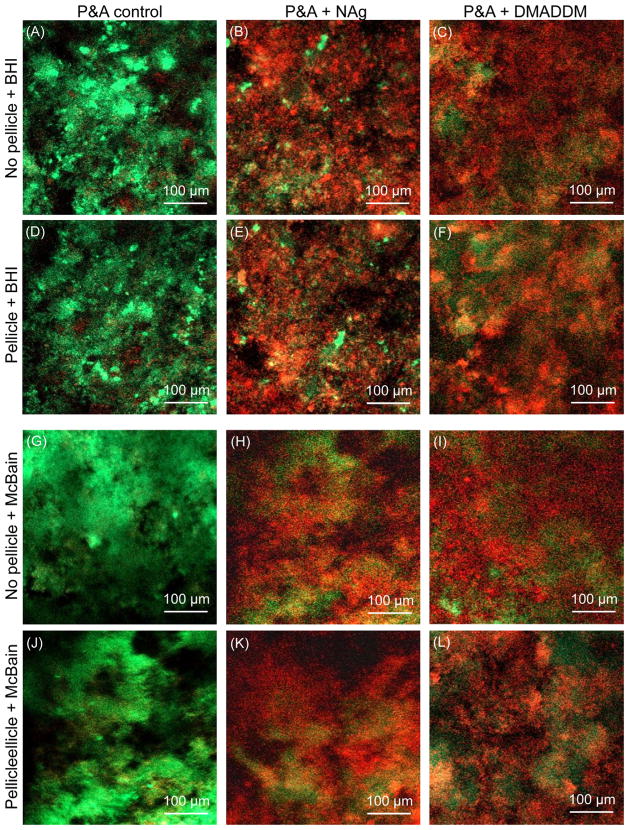
Representative live/dead staining CLSM images are shown in Fig. 1 for the adherent biofilms on resin disks. The control adhesive disks were covered with primarily live bacteria. In contrast, adhesive disks containing DMADDM or NAg had much more staining of compromised bacteria, when cultured in both BHI and McBain medium, even for specimens pre-covered with salivary pellicles.
Fig. 1.
CLSM images of live/dead biofilms on resin. The top titles indicate the bonding agents, and the left titles indicate whether the resin was pre-coated with human salivary pellicles, and the culture medium type. Bacteria with integral membrane were stained green, and bacteria with compromised membrane were stained red. Bacteria of those two cohorts that were close to or on the top of each other produced orange or yellow colors. It should be noted that some red cells may have partially lost membrane function and may still be viable. Control disks were covered with primarily live bacteria. Adhesives containing DMADDM or NAg greatly inhibited the microcosm biofilm growth.
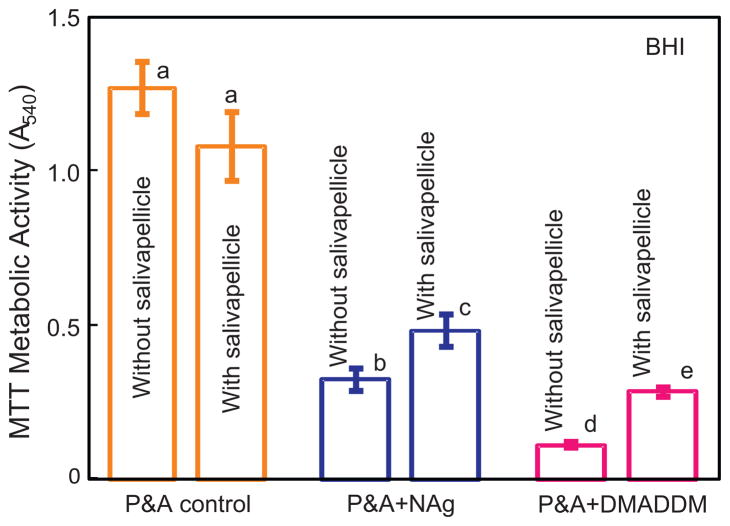
MTT metabolic activity with culture in BHI medium is plotted in Fig. 2 (mean ± sd; n = 6). Comparing between the three materials, P&A control had the highest MTT absorbance, followed by P&A + NAg; P&A + DMADDM had the strongest antibacterial activity with the lowest metabolic activity (p < 0.05). The control showed no significant difference with or without salivary pellicle (p > 0.1). For P&A + NAg and P&A + DMADDM, pre-treating the resin surface with salivary pellicles significantly increased the metabolic activity of biofilms, compared to bare resin surface counterparts (p < 0.01). These results show that adding NAg or DMADDM in the bonding agents imparted a strong antibacterial activity; however, pre-coating the resin surface with salivary pellicles significantly decreased the antibacterial effect against biofilms, when cultured in BHI.
Fig. 2.
MTT assay of metabolic activity of biofilms on resin disks cultured in BHI (mean ± sd; n = 6) for P&A control, P&A + NAg, and P&A + DMADDM. “P” stands for the commercial SBMP primer control, and “A” stands for SBMP adhesive. A higher MTT absorbance indicates a higher formazan concentration, which in turn indicates more metabolic activity in the biofilm. Values with dissimilar letters are significantly different from each other (p < 0.05).
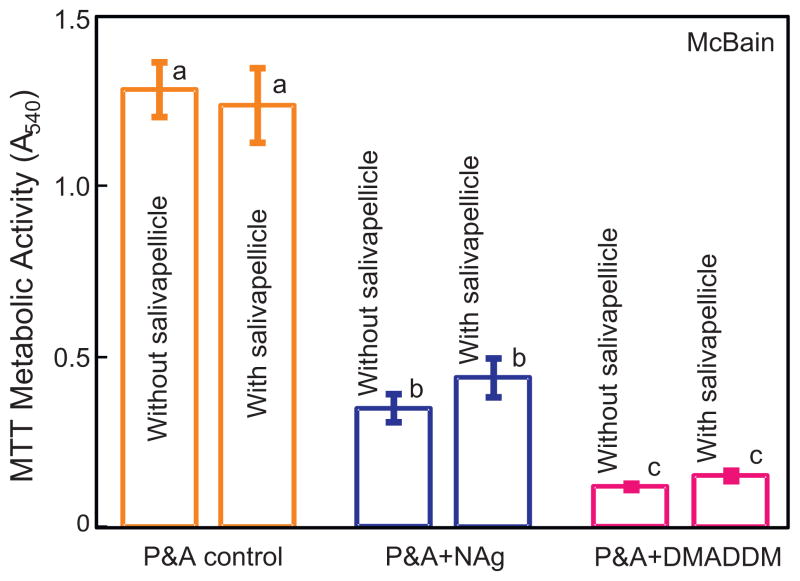
Fig. 3 shows the MTT metabolic activity of biofilms when cultured in the saliva-like McBain medium. Adding NAg into bonding agent yielded a strong antibacterial activity; and adding DMADDM produced an even stronger antibacterial activity (p < 0.05). Without pre-coating of salivary pellicles, the incorporation of NAg or DMADDM decreased the MTT activity of biofilms to 27% and 9% of that of the control, respectively. For each material, there was no significant difference with or without pre-coating of salivary pellicles (p > 0.1) when cultured in the McBain medium.
Fig. 3.
MTT metabolic activity of biofilms when cultured in the saliva-like McBain medium (mean ± sd; n = 6) for P&A control, P&A + NAg, and P&A + DMADDM. Adding NAg into bonding agent yielded a strong antibacterial activity; and adding DMADDM produced an even stronger antibacterial activity (p < 0.05). Values with dissimilar letters are significantly different from each other (p < 0.05).
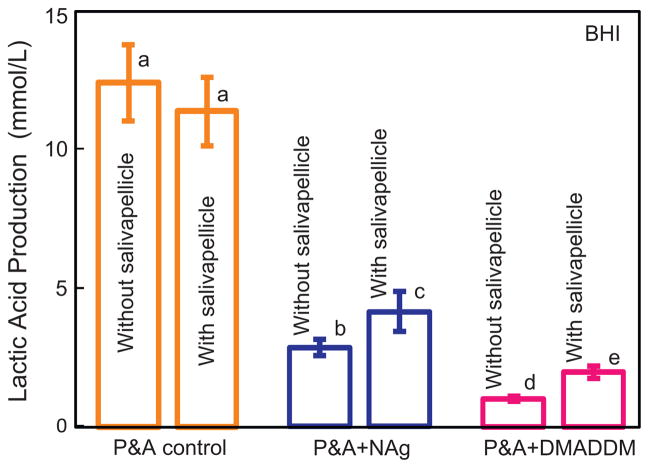
The lactic acid production by biofilms adherent on the resin disks is plotted in Fig. 4 using the BHI medium. Without salivary pellicle coverage, the biofilms on P&A control produced the most acid (p < 0.05), followed by P&A + NAg (with 23% of the acid of the control), and P&A + DMADDM (with 8% of the acid of the control). After salivary pellicle treatment, the incorporation of NAg or DMADDM decreased the acid production to 36% and 17% of the control (p < 0.05), respectively. For both P&A + NAg and P&A + DMADDM, salivary pellicle treatment significantly increased the lactic acid production (p < 0.05).
Fig. 4.
Lactic acid production by biofilms on resin disks in BHI medium (mean ± sd; n = 6) for P&A control, P&A + NAg, and P&A + DMADDM. Values with dissimilar letters are significantly different from each other (p < 0.05).
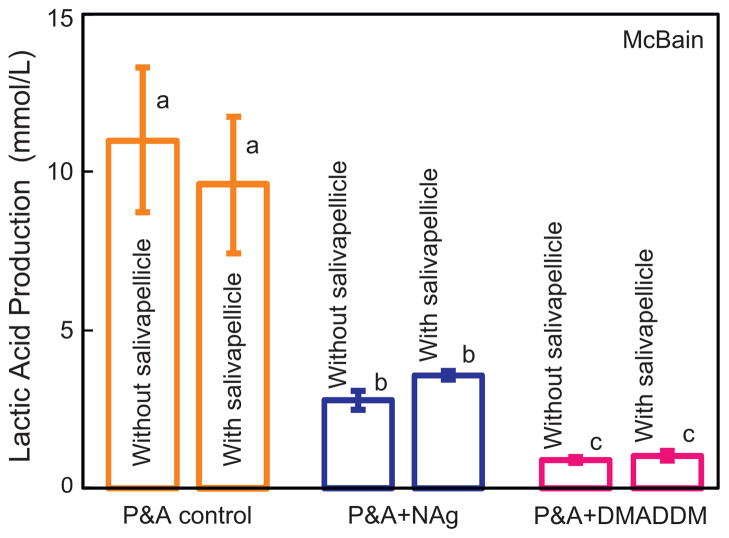
Fig. 5 plots the lactic acid production by biofilm on resin disks cultured in McBain medium. Without salivary pre-coating on the resin, the biofilms on P&A control produced the most acid (p < 0.05), followed by P&A + NAg (with 24% of the acid of control), and P&A + DMADDM (with 8% of the acid of control). After pre-coating with salivary pellicles, the incorporation of NAg or DMADDM decreased the acid production to 33% and 11% of that of control (p < 0.05), respectively. For every material, salivary pellicle pre-treatment had no significant effect on acid production when using the McBain medium (p > 0.1).
Fig. 5.
Lactic acid production by biofilm on resin disks cultured in McBain medium (mean ± sd; n = 6) for P&A control, P&A + NAg, and P&A + DMADDM. The incorporation of NAg or DMADDM greatly decreased the acid production of the adherent biofilms. Values with dissimilar letters are significantly different from each other (p < 0.05).
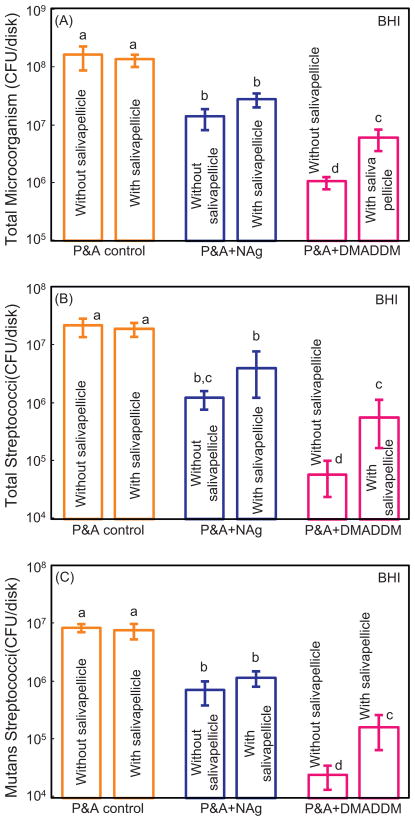
CFU counts of biofilms adherent on resin disks are plotted in Fig. 6 for culture in BHI medium. Without salivary treatment, adding NAg or DMADDM in adhesive greatly decreased the biofilm CFU, compared to control (p < 0.05). P&A + DMADDM showed the strongest antibacterial effect, reducing the CFU by more than two orders of magnitude (p < 0.05). For P&A + NAg, the salivary pellicle pre-coating slightly, but not significantly, increased the CFU values (p > 0.1). For P&A + DMADDM, pre-coating with salivary pellicles yielded significantly higher CFU, by nearly an order of magnitude, than the counterpart without pre-coating of salivary pellicles (p < 0.05).
Fig. 6.
Colony-forming unit (CFU) counts of biofilms in BHI medium for P&A control, P&A + NAg, and P&A + DMADDM (mean ± sd; n = 6). Three types of agar plates were tested: (A) Total microorganisms; (B) total streptococci; and (C) mutans streptococci. For each type of agar plates, values with dissimilar letters are significantly different from each other (p < 0.05). Adding NAg or DMADDM in adhesive greatly decreased the biofilm CFU, compared to control (p < 0.05).
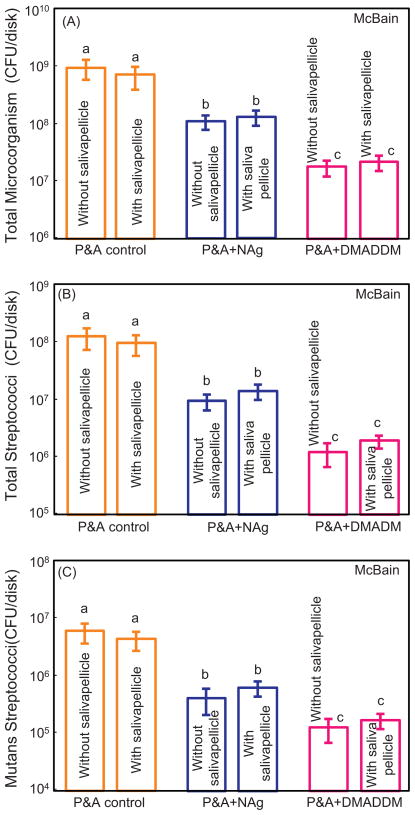
CFU counts of biofilms cultured in McBain medium are plotted in Fig. 7. For each material, with or without pre-coating of salivary pellicle had no significant effect on CFU (p > 0.1). The incorporation of NAg or DMADDM in adhesive greatly decreased the CFU, compared to the control (p < 0.05). P&A + DMADDM showed the strongest antibacterial effect, reducing the CFU values by almost two orders of magnitude, compared to the commercial control adhesive group (p < 0.05).
Fig. 7.
CFU counts of biofilms cultured in McBain medium for P&A control, P&A + NAg, and P&A + DMADDM (mean ± sd; n = 6). Three types of agar plates were tested: (A) Total microorganisms; (B) total streptococci; and (C) mutans streptococci. For each type of agar plates, values with dissimilar letters are significantly different from each other (p < 0.05). The incorporation of NAg or DMADDM in adhesive substantially decreased the CFU, compared to the control (p < 0.05).
4. Discussion
In the present study, a new antibacterial monomer DMADDM and NAg were incorporated into a bonding agent, and the antibacterial efficacy of the adhesive specimens against salivary protein pellicles was investigated for the first time. The bonding agents containing DMADDM and NAg greatly inhibited dental plaque microcosm biofilm growth, metabolic activity, CFU and lactic acid production, even when the resin surface was pre-coated with salivary pellicles. Previous studies have used oral biofilm models which can be divided into three categories: single species, defined consortium, and microcosm [43]. Dental plaque is a complicated ecosystem with about 1000 bacterial species [42], therefore the use of a microcosm model would be preferred to maintain the complexity and heterogeneity of biofilms in vivo [43]. Oral bacteria in vivo colonize on the tooth-restoration surfaces to form biofilms, and cariogenic bacteria in the biofilms can metabolize carbohydrates to organic acids. This in turn leads to tooth decay and secondary caries at the tooth-restoration margins. Therefore, the new DMADDM- and NAg-containing antibacterial bonding agents with substantial reductions in biofilm growth and lactic acid production are promising for caries-inhibition applications in the oral environment filled with saliva.
Previous investigations reported the contact-inhibition of antibacterial resins containing quaternary ammonium methacrylates [19–25], such as MDPB-containing materials [20,46] and DMAE-CB-incorporated adhesive [21,37]. The quaternary ammonium methacrylates can be immobilized in the resin by covalent bonding during co-polymerization. Hence, the mode of antibacterial action is contact-inhibition. Previous studies suggested that when the negatively-charged bacterial cell contacts the positively-charged sites of QAS resin, the electric balance of cell membrane could be disturbed, and the bacterium could explode under its own osmotic pressure [52,53]. This contact-killing mechanism would imply that, when a salivary protein pellicle separates the antibacterial resin surface from the overlaying biofilm, the antibacterial effect of the resin could be decreased.
Indeed, several publications showed that a saliva-derived protein film on the cationic antibacterial surfaces reduced the original bactericidal effect [45–47]. The MDPB immobilized fillers, which contained MDPB at a concentration of 15.8%, could greatly inhibit the bacterial growth; however, saliva pre-treatment of the surface reduced its antibacterial potency [46]. After incorporating the MDPB fillers into a composite at a final concentration of 2.83% of MDPB, the composite could substantially suppress Streptococcus mutans biofilm accumulation [45]. However, this anti-plaque ability was reduced by salivary pre-treatment of the composite surface, although the composite still exhibited significantly less plaque accumulation on its surface than a control composite [45]. Therefore, the reduction in the antibacterial effect by the adsorption of proteins on the resin surfaces has been considered a drawback of contact-killing dental materials.
Promising results were obtained in the present study using the newly-developed DMADDM and NAg in bonding agents. When cultured in the BHI medium without salivary proteins, the new antibacterial bonding agents greatly reduced biofilm activities. Covering with salivary pellicles indeed reduced the antibacterial effect. However, the bonding agents containing DMADDM and NAg still substantially inhibited biofilm activities even with salivary pellicles. Furthermore, when cultured in the in artificial saliva McBain medium, the new antibacterial bonding agents containing DMADDM and NAg showed no decrease in antibacterial efficacy even with salivary pellicle coverage.
The present study used an artificial saliva for biofilm culture, the McBain medium, which reflects the gross composition of salivary secretions except certain antimicrobial peptides and hydrolytic enzymes present within the mouth [49]. One major component of this medium, mucin, accounts for up to 26% of the natural salivary proteins [54] and is an important salivary protein in the pellicle [44]. Mucin and other proteins in the McBain medium could be absorbed onto the resin to form a medium-derived pellicle, which could cover the anti-biofilm sites of the cationic resin surface, thereby reducing its antibacterial effect. This was likely why, when cultured in McBain medium, coating an additional human salivary pellicle to the resin surface did not further decrease its antibacterial effect.
In this regard, the use of BHI and other culture mediums without salivary proteins, together with the use of antibacterial resins without pre-coating of salivary pellicles, would yield artificially high antibacterial effects. Such antibacterial activities could greatly diminish once the resin surfaces are coated with salivary pellicles when placed in vivo. On the other hand, the use of saliva-mimicking culture mediums such as the McBain medium could reasonably well measure the antibacterial efficacy of the resin, because it produces a medium-derived pellicle. This yields similar antibacterial properties for the resin with or without salivary pellicle coating, indicating that the resin covered with medium-derived pellicle could be equivalent to human salivary pellicle-coated resin in antibacterial measurements. Therefore, the microcosm biofilm model together with the artificial saliva medium of the present study could be an appropriate tool to monitor the contact-killing mode of antibacterial behavior of QAS-incorporated materials.
The incorporation of NAg at a low concentration of 0.1% also provided a strong antibacterial activity. NAg was prepared by dissolving the Ag salt in TBAEMA monomer, which was then mixed with the resin and photopolymerized, thus forming the NAg in situ. This yielded well-dispersed NAg in the resin without agglomeration, and avoided the need to mix and disperse nanoparticles in resins [39–41]. The small particle size of NAg, about 2.7 nm in diameter, yielded a high specific surface area, which allowed the use of a low NAg filler level in the adhesive while achieving a strong antibacterial effect. Considering the cured bonding agent with NAg being covered with a salivary pellicle, it is possible that the Ag ions could diffuse and penetrate through the salivary pellicle, and hence exert antibacterial effects which may not be impeded by the salivary pellicles. However, when the effect of salivary pellicle on NAg-containing material was measured in the present study, it was found that its antibacterial activity significantly decreased due to salivary pellicles when cultured in BHI medium. This may be due to the antibacterial mode of NAg-containing materials which involves Ag ion release. Ag ions could target the thiol groups (-SH) of proteins exposed to the extracellular portion of the bacterial membrane [55–57] and inactivate the vital enzymes of bacteria, thus causing DNA in the bacteria to lose its replication ability, which leads to cell death [58]. However, when covered with salivary pellicles, the negatively-charged N-terminal domain of the acidic proline-RICH proteins (PRPs), which comprise 37% of the salivary proteins adhering to freshly cleaned teeth [59], could capture the positively charged Ag ions and work as barrier to hinder Ag+ ion release. This was likely why the antibacterial effect of the NAg-containing material decreased after salivary pellicle treatment when cultured in BHI medium. However, even with salivary pellicles, the bonding agents with NAg and DMADDM still greatly reduced biofilm growth and acid production, compared to a commercial bonding agent. Furthermore, recent studies showed that the incorporation of DMADDM and NAg into bonding agents did not adversely affect the dentin bond strength [48]. Therefore, DMADDM and NAg are promising for use in primers and adhesives to inhibit the accumulation of biofilms and plaque and the resultant secondary caries. Further studies are needed to investigate their efficacy in vivo.
5. Conclusions
Antibacterial bonding agents containing novel DMADDM and NAg were synthesized which greatly reduced the metabolic activity, CFU and lactic acid of microcosm biofilms on resin surfaces, even when pre-coated with salivary pellicles. When cultured in a medium without salivary proteins, the pre-coating of salivary pellicles on the resin surface significantly decreased its antibacterial efficacy, compared to resin without pellicles. However, when cultured in an artificial saliva medium, the resins with or without the pre-coating of salivary pellicles had similar antibacterial effects. The results indicate that saliva-like culture mediums or pre-coating the resin with salivary pellicles are needed to avoid overestimating the antibacterial efficacy. The results further indicate that the novel DMADDM- and NAg-containing bonding agents could greatly inhibit biofilms even with salivary pellicles, and hence are promising for clinical applications in antibacterial and anti-caries restorations.
Acknowledgments
We thank Dr. Lei Cheng and Dr. Ke Zhang of the University of Maryland School of Dentistry, and Dr. Joseph M. Antonucci, Dr. Nancy J. Lin and Dr. Sheng Lin-Gibson of the National Institute of Standards and Technology for fruitful discussions. This study was supported by NIH R01 DE17974 (HX), National Natural Science Foundation of China 81100772 (FL), and University of Maryland School of Dentistry Bridging Fund (HX).
References
- 1.Bayne SC, Thompson JY, Swift EJ, Jr, Stamatiades P, Wilkerson M. A characterization of first-generation flowable composites. J Am Dent Assoc. 1998;129:567–77. doi: 10.14219/jada.archive.1998.0274. [DOI] [PubMed] [Google Scholar]
- 2.Lim BS, Ferracane JL, Sakaguchi RL, Condon JR. Reduction of polymerization contraction stress for dental composites by two-step light-activation. Dent Mater. 2002;18:436–44. doi: 10.1016/s0109-5641(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 3.Watts DC, Marouf AS, Al-Hindi AM. Photo-polymerization shrinkage-stress kinetics in resin-composites: methods development. Dent Mater. 2003;19:1–11. doi: 10.1016/s0109-5641(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 4.Fruits TJ, Knapp JA, Khajotia SS. Microleakage in the proximal walls of direct and indirect posterior resin slot restorations. Oper Dent. 2006;31:719–27. doi: 10.2341/05-148. [DOI] [PubMed] [Google Scholar]
- 5.Coelho-De-Souza FH, Camacho GB, Demarco FF, Powers JM. Fracture resistance and gap formation of MOD restorations: influence of restorative technique, bevel preparation and water storage. Oper Dent. 2008;33:37–43. doi: 10.2341/07-27. [DOI] [PubMed] [Google Scholar]
- 6.Samuel SP, Li S, Mukherjee I, Guo Y, Patel AC, Baran G, et al. Mechanical properties of experimental dental composites containing a combination of mesoporous and nonporous spherical silica as fillers. Dent Mater. 2009;25:296–301. doi: 10.1016/j.dental.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. J Dent Res. 2008;87:710–9. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Godoy F, Kramer N, Feilzer AJ, Frankenberger R. Long-term degradation of enamel and dentin bonds: 6-year results in vitro vsin vivo. Dent Mater. 2010;26:1113–8. doi: 10.1016/j.dental.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Ferracane JL. Resin composite – state of the art. Dent Mater. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Demarco FF, Correa MB, Cenci MS, Moraes RR, Opdam NJM. Longevity of posterior composite restorations: not only a matter of materials. Dent Mater. 2012:87–101. doi: 10.1016/j.dental.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Prim Dent Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 12.National Institute of Dental and Craniofacial Research (NIDCR) announcement # 13-DE-102. Dental Resin Composites and Caries. 2009 Mar 5; [Google Scholar]
- 13.Zalkind MM, Keisar O, Ever-Hadani P, Grinberg R, Sela MN. Accumulation of Streptococcus mutans on light-cured composites and amalgam: an in vitro study. J Esthet Dent. 1998;10:187–90. doi: 10.1111/j.1708-8240.1998.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 14.Beyth N, Domb AJ, Weiss EI. An in vitro quantitative antibacterial analysis of amalgam and composite resins. J Dent. 2007;35:201–6. doi: 10.1016/j.jdent.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Loguercio AD, Reis A, Bortoli G, Patzlaft R, Kenshima S, Kenshima S, et al. Influence of adhesive systems on interfacial dentin gap formation in vitro. Oper Dent. 2006;31:431–41. doi: 10.2341/05-53. [DOI] [PubMed] [Google Scholar]
- 16.Awliya WY, El-Sahn AM. Leakage pathway of Class V cavities restored with different flowable resin composite restorations. Oper Dent. 2008;33:31–6. doi: 10.2341/07-22. [DOI] [PubMed] [Google Scholar]
- 17.Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. Quality of dental restorations. FDI Commission Project 2-95. Int Dent J. 2001;51:117–58. doi: 10.1002/j.1875-595x.2001.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 18.Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Dent Mater; Summary of discussion from the Portland Composites Symposium (POCOS); June 17–19, 2004; Portland, Oregon: Oregon Health and Science University; 2005. pp. 3–6. [DOI] [PubMed] [Google Scholar]
- 19.Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RR. Incorporation of bacterial inhibitor into resin composite. J Dent Res. 1994;73:1437–43. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- 20.Imazato S, Ehara A, Torii M, Ebisu S. Antibacterial activity of dentine primer containing MDPB after curing. J Dent. 1998;26:267–71. doi: 10.1016/s0300-5712(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 21.Li F, Chai ZG, Sun MN, Wang F, Ma S, Zhang L, et al. Anti-biofilm effect of dental adhesive with cationic monomer. J Dent Res. 2009;88:372–6. doi: 10.1177/0022034509334499. [DOI] [PubMed] [Google Scholar]
- 22.Xie D, Weng Y, Guo X, Zhao J, Gregory RL, Zheng C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dent Mater. 2011;27:487–96. doi: 10.1016/j.dental.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent Mater. 2012;28:219–28. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng L, Weir MD, Xu HH, Antonucci JM, Kraigsley AM, Lin NJ, et al. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent Mater. 2012;28:561–72. doi: 10.1016/j.dental.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X, Wang Y, Liao S, Wen ZT, Fan Y. Synthesis and characterization of antibacterial dental monomers and composites. J Biomed Mater Res B. 2012;100:1151–62. doi: 10.1002/jbm.b.32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thome T, Mayer MP, Imazato S, Geraldo-Martins VR, Marques MM. In vitro analysis of inhibitory effects of the antibacterial monomer MDPB-containing restorations on the progression of secondary root caries. J Dent. 2009;37:705–11. doi: 10.1016/j.jdent.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Weng Y, Howard L, Guo X, Chong VJ, Gregory RL, Xie D. A novel antibacterial resin composite for improved dental restoratives. J Mater Sci Mater Med. 2012;23:1553–61. doi: 10.1007/s10856-012-4629-z. [DOI] [PubMed] [Google Scholar]
- 28.Cheng L, Weir MD, Zhang K, Xu SM, Chen Q, Zhou XD, et al. Antibacterial nanocomposite with calcium phosphate and quaternary ammonium. J Dent Res. 2012;91:460–6. doi: 10.1177/0022034512440579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res. 2002;62:447–56. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 30.Ikemura K, Tay FR, Endo T, Pashley DH. A review of chemical-approach and ultramorphological studies on the development of fluoride-releasing dental adhesives comprising new pre-reacted glass ionomer (PRG) fillers. Dent Mater J. 2008;27:315–29. doi: 10.4012/dmj.27.315. [DOI] [PubMed] [Google Scholar]
- 31.Ritter AV, Swift EJ, Jr, Heymann HO, Sturdevant JR, Wilder AD., Jr An eight-year clinical evaluation of filled and unfilled one-bottle dental adhesives. J Am Dent Assoc. 2009;140:28–37. doi: 10.14219/jada.archive.2009.0015. [DOI] [PubMed] [Google Scholar]
- 32.Park J, Eslick J, Ye Q, Misra A, Spencer P. The influence of chemical structure on the properties in methacrylate-based dentin adhesives. Dent Mater. 2011;27:1086–93. doi: 10.1016/j.dental.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pashley DH, Tay FR, Breschi L, Tjaderhane L, Carvalho RM, Carrilho M, et al. State of the art etch-and-rinse adhesives. Dent Mater. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J. State of the art of self-etch adhesives. Dent Mater. 2011;27:17–28. doi: 10.1016/j.dental.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 35.Pinzon LM, Powers JM, O’Keefe KL, Dusevish V, Spencer P, Marshall GW. Effect of mucoprotein on the bond strength of resin composite to human dentin. Odontology. 2011;99:119–28. doi: 10.1007/s10266-011-0002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imazato S, Kuramoto A, Takahashi Y, Ebisu S, Peters MC. In vitro antibacterial effects of the dentin primer of Clearfil Protect Bond. Dent Mater. 2006;22:527–32. doi: 10.1016/j.dental.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Li F, Chen J, Chai Z, Zhang L, Xiao Y, Fang M, et al. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J Dent. 2009;37:289–96. doi: 10.1016/j.jdent.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Fan C, Chu L, Rawls HR, Norling BK, Cardenas HL, Whang K. Development of an antimicrobial resin – a pilot study. Dent Mater. 2011;27:322–8. doi: 10.1016/j.dental.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Cheng YJ, Zeiger DN, Howarter JA, Zhang X, Lin NJ, Antonucci JM, et al. In situ formation of silver nanoparticles in photocrosslinking polymers. J Biomed Mater Res B. 2011;97:124–31. doi: 10.1002/jbm.b.31793. [DOI] [PubMed] [Google Scholar]
- 40.Cheng L, Zhang K, Melo MA, Weir MD, Zhou X, Xu HH. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. J Dent Res. 2012;91:598–604. doi: 10.1177/0022034512444128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang K, Melo MAS, Cheng L, Weir MD, Bai YX, Xu HHK. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dent Mater. 2012;28:842–52. doi: 10.1016/j.dental.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ten Cate JM. Biofilms, a new approach to the microbiology of dental plaque. Odontology. 2006;94:1–9. doi: 10.1007/s10266-006-0063-3. [DOI] [PubMed] [Google Scholar]
- 43.McBain AJ. In vitro biofilm models: an overview. Adv Appl Microbiol. 2009;69:99–132. doi: 10.1016/S0065-2164(09)69004-3. [DOI] [PubMed] [Google Scholar]
- 44.Lendenmann U, Grogan J, Oppenheim FG. Saliva and dental pellicle – a review. Adv Dent Res. 2000;14:22–8. doi: 10.1177/08959374000140010301. [DOI] [PubMed] [Google Scholar]
- 45.Ebi N, Imazato S, Noiri Y, Ebisu S. Inhibitory effects of resin composite containing bactericide-immobilized filler on plaque accumulation. Dent Mater. 2001;17:485–91. doi: 10.1016/s0109-5641(01)00006-9. [DOI] [PubMed] [Google Scholar]
- 46.Imazato S, Ebi N, Takahashi Y, Kaneko T, Ebisu S, Russell RR. Antibacterial activity of bactericide-immobilized filler for resin-based restoratives. Biomaterials. 2003;24:3605–9. doi: 10.1016/s0142-9612(03)00217-5. [DOI] [PubMed] [Google Scholar]
- 47.Muller R, Eidt A, Hiller KA, Katzur V, Subat M, Schweikl H, et al. Influences of protein films on antibacterial or bacteria-repellent surface coatings in a model system using silicon wafers. Biomaterials. 2009;30:4921–9. doi: 10.1016/j.biomaterials.2009.05.079. [DOI] [PubMed] [Google Scholar]
- 48.Cheng L, Weir MD, Zhang K, Arola DD, Zhou X, Xu HHK. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J Dent. 2012 doi: 10.1016/j.jdent.2013.01.004. (submitted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McBain AJ, Sissons C, Ledder RG, Sreenivasan PK, De Vizio W, Gilbert P. Development and characterization of a simple perfused oral microcosm. J Appl Microbiol. 2005;98:624–34. doi: 10.1111/j.1365-2672.2004.02483.x. [DOI] [PubMed] [Google Scholar]
- 50.Shewmaker PL, Gertz RE, Jr, Kim CY, de Fijter S, DiOrio M, Moore MR, et al. Streptococcus salivarius meningitis case strain traced to oral flora of anesthesiologist. J Clin Microbiol. 2010;48:2589–91. doi: 10.1128/JCM.00426-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lima JP, Sampaio de Melo MA, Borges FM, Teixeira AH, Steiner-Oliveira C, Nobre Dos Santos M, et al. Evaluation of the antimicrobial effect of photodynamic antimicrobial therapy in an in situ model of dentine caries. Eur J Oral Sci. 2009;117:568–74. doi: 10.1111/j.1600-0722.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- 52.Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 2006;27:3995–4002. doi: 10.1016/j.biomaterials.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Namba N, Yoshida Y, Nagaoka N, Takashima S, Matsuura-Yoshimoto K, Maeda H, et al. Antibacterial effect of bactericide immobilized in resin matrix. Dent Mater. 2009;25:424–30. doi: 10.1016/j.dental.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 54.Amerongen AV, Bolscher JG, Veerman EC. Salivary mucins: protective functions in relation to their diversity. Glycobiology. 1995;5:733–40. doi: 10.1093/glycob/5.8.733. [DOI] [PubMed] [Google Scholar]
- 55.Gupta A, Matsui K, Lo JF, Silver S. Molecular basis for resistance to silver cations in Salmonella. Nat Med. 1999;5:183–8. doi: 10.1038/5545. [DOI] [PubMed] [Google Scholar]
- 56.Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52:662–8. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 57.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–53. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 58.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Bennick A, Chau G, Goodlin R, Abrams S, Tustian D, Madapallimattam G. The role of human salivary acidic proline-rich proteins in the formation of acquired dental pellicle in vivo and their fate after adsorption to the human enamel surface. Arch Oral Biol. 1983;28:19–27. doi: 10.1016/0003-9969(83)90022-5. [DOI] [PubMed] [Google Scholar]