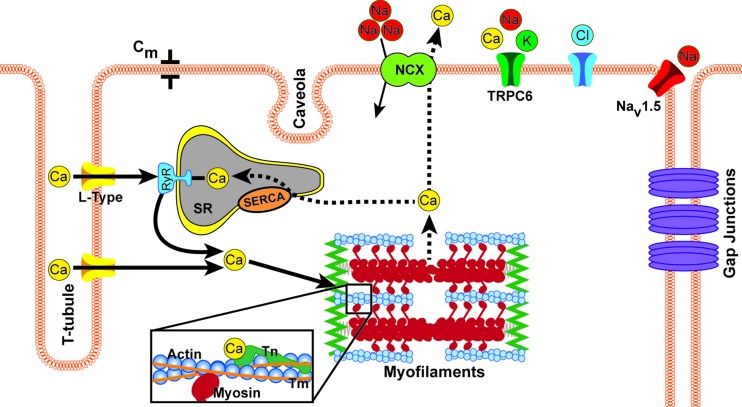
Fig. 2.
The first step in the initiation of contraction begins with an influx of sodium ions, which depolarizes the membrane and opens the voltage-gated L-type calcium channels. This causes an influx of calcium into the cell, some of which binds to ryanodine receptors (RyR) located on surface of the sarcoplasmic reticulum (SR), which allows for a large scale release of calcium from inside the SR; a process referred to as calcium induced calcium release. There is then an abundance of free calcium in the cell that can bind to troponin (Tn), in particular troponin-C. This binding causes tropomyosin (Tm) to shift, exposing the myosin binding site on actin. Once the myosin head binds to actin, force is generated. At the end of the crossbridge cycle, calcium is released from troponin-C and is then either pumped out of the cell by the sodium-calcium exchanger (NCX) or resequestered into the SR via the sarcoplasmic reticulum calcium ATPase (SERCA) pump. Resulting changes in the mechanical context of the cell can alter the dynamics of conduction of electrical excitation throughout the tissue and the duration of cell action potential, by modulating channels, junctions, and cell capacitances and resistances; thus feeding back between cardiac mechanics and electrophysiology.