Abstract
Genetic factors that influence seizure susceptibility can act transiently during the development of neural circuits or might be necessary for the proper functioning of existing circuits. We provide evidence that the Drosophila seizure-sensitive mutant easily shocked (eas) represents a neurological disorder in which abnormal functioning of existing neural circuits leads to seizure sensitivity. The eas+ gene encodes for the protein Ethanolamine Kinase, involved in phospholipid biosynthesis. We show that induction of eas+ in adult mutant flies rescues them from seizure sensitivity despite previously known developmental defects in brain morphology. Additionally, through cell-type-specific rescue, our results suggest a specific role for eas+ in excitatory rather than inhibitory neural transmission. Overall, our findings emphasize an important role for proper phospholipid metabolism in normal brain function and suggest that certain classes of epilepsy syndromes could have the potential to be treated with gene therapy techniques.
INDEXING TERMS: epilepsy, seizure, ethanolamine kinase, phospholipids, acute, cholinergic
Genetic mutations causing defects in the nervous system can give rise to neural hyperexcitability and epilepsy by altering the balance of excitatory and inhibitory circuits. Various classes of mutations can cause hyperexcitability: those that transiently disrupt the development of neural circuits or those that interfere with normal function of the established nervous system. Distinguishing between strictly developmental or chronic physiological deficiencies that lead to high seizure susceptibilities will be necessary for understanding what cellular processes contribute to the increased excitability of the nervous system and may guide treatments to reduce seizures.
Defects in neural development can alter the contributions of excitatory and inhibitory circuits. For example, in transgenic mice, mutations in the human epilepsy gene leucine-rich glioma-inactivated protein-1, or LGI1, caused impaired postnatal pruning and development of glutaminergic circuits within the CNS. This led to increased excitatory synaptic transmission while leaving inhibition unaffected, indicating that treatment has to occur during a specific time window of development to rescue the original molecular deficit (Zhou et al., 2009). In contrast, certain mutations could acutely alter the activity of existing neural circuits and act independently of developmental processes. Mutations of the human voltage-gated sodium channel Nav1.1 (SCN1a) that affect Na+ current have been associated with Dravet’s syndrome (SMEI; severe myoclonic epilepsy of infants) and GEFS+ (generalized epilepsy febrile seizures plus; Claes et al., 2001; Yu et al., 2006). A knock-in genetic model for GEFS+ in Drosophila leads to decreased activity of GABAergic neurons, suggesting imbalances in inhibitory activity in the nervous system (Sun et al., 2012).
Drosophila is an excellent model system for studying the molecular mechanisms underlying seizure disorders, because genetic techniques allow the precise control of gene expression temporally and spatially (Brand and Perrimon, 1993). Additionally, a large collection of fly mutants display a wide range of seizure susceptibilities (for review see Parker et al., 2011a). The easily shocked (eas) mutation represents a recessive loss-of-function mutation in the gene encoding an ethanolamine kinase, the first enzyme in the CDP-ethanolamine branch of the Kennedy pathway leading to the synthesis of the phospholipid phosphotidylethanolamine (PtdEtn; Pavlidis et al., 1994).
To investigate the temporal requirements of eas+ expression for rescue from seizure susceptibility, we expressed the eas+ gene in adult flies and tracked their seizure behavior over time. Expression of the gene postdevelopmentally was sufficient for rescue from the seizure-sensitive phenotype. Additionally, we found evidence that excitatory rather than inhibitory transmission is affected by the eas mutation. Therefore, the seizure disorder in the eas mutant appears to result from acute physiological defects that influence seizure susceptibility through excitatory circuits within the nervous system.
MATERIALS AND METHODS
Fly stocks
Flies and crosses were maintained on standard cornmeal molasses medium at 25°C in a dark incubator. The null eas allele used in this study is eas2, also known as easPC80 (Pavlidis et al., 1994). The sda and parabss1 seizure-sensitive mutants have been described by Song et al. (2008). The P[w+ hs-c12] transgene (hereafter referred to as hs-eas+) was originally described by Pavlidis et al. (1994). The UAS-eas+ transgene was obtained from Thomas Préat (Pascual et al., 2005). GAL4 stocks were obtained from Bloomington Stock Center.
Bang-sensitivity assay
Bang-sensitivity tests were performed as described by Song et al. (2008). Up to 25 flies were collected under CO2, placed in a fresh food vial, and allowed to recover overnight. For testing, the vial was stimulated with a VWR Vortex Mixer (VWR International, West Chester, PA) at maximum speed for 10 seconds. The bang-sensitive (BS) phenotype is scored by the number of flies that experience temporary paralysis (~20–40 seconds) over the total number of flies tested. Pools of flies were combined.
Heat shock regimen
Virgin eas flies were crossed to hs-eas+ males, generating male eas; hs-eas+/+ flies. Progeny 0–2 days after eclosion were subjected to a multiday heat shock regimen. Each day for up to 5 continuous days, groups of flies were tested for bang sensitivity (by 10-second vortex), followed by a 2-hour heat shock in a 37°C water bath. Only one heat shock was given per day. After each heat shock, flies were allowed to recover overnight at 25°C. Food vials were changed approximately every 2 days. Flies were tested either for bang sensitivity (by 10-second vortex) or seizure susceptibility via electrophysiology approximately 22 hours after the last heat shock treatment.
GAL4/UAS crosses
Virgin eas flies with the GAL4 transgene were crossed to UAS-eas+ male flies, and male eas mutant progeny were collected. All behavioral and electrophysiological experiments on transgenic GAL4/UAS flies were performed with 1–2-day-old males.
Electrophysiological seizure-susceptibility assays
Methods for electrophysiological testing of seizure susceptibility via the dorsal longitudinal muscle (DLM) has been described by Kuebler and Tanouye (2000), Song and Tanouye (2006) and Lee and Wu (2006). A vacuum line was used to manipulate the fly without the aid of CO2 or any anesthetic. Flies were mounted in a channel made of wax on a glass microscope slide, leaving the dorsal head, thorax, and abdomen exposed. Next, electrodes (made of uninsulated tungsten) were inserted into the fly. Stimulating electrodes were placed into the brain, recording electrode placed into the DLM, and ground electrode into the abdomen. Seizure-like activity was evoked by high-frequency (HF) brain stimulation (0.5-msec pulses at 200 Hz for 300 msec), and seizures were monitored via the activity of the DLM muscle. To determine seizure thresholds, HF stimuli were first given to flies at an intensity predicted by their behavior. If the stimulus failed to elicit a seizure, the intensity was increased until a seizure was induced. The threshold was determined for an individual fly as the lowest intensity at which seizures occurred. Between HF stimulation, the fly was allowed to recover for at least 10 minutes. Throughout the course of the experiments, the giant fiber (GF) circuit was monitored using single pulses (0.2-msec duration, 0.5 Hz) to ensure normal response of the GF circuit.
RESULTS
Rescue of the seizure-sensitive phenotype in adults
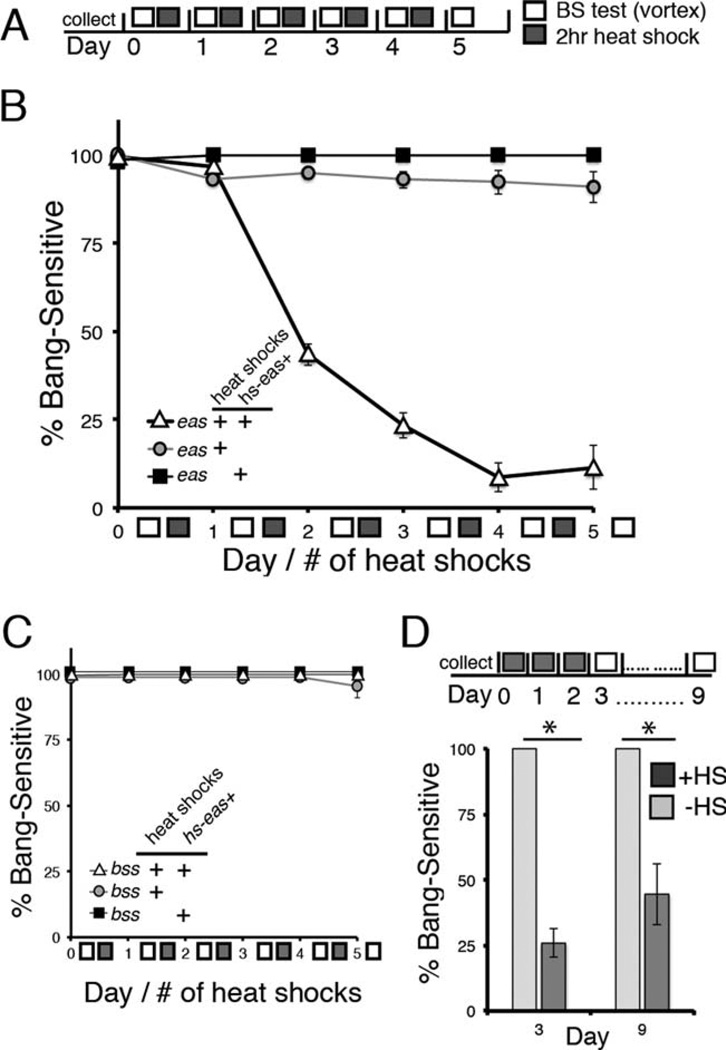
Upon vigorous mechanical stimulation, 100% of eas mutants display an abnormal bang-sensitive (BS) behavioral phenotype that is an indication of severe seizure sensitivity, whereas wild-type flies never exhibit this behavior. To determine when expression of the eas+ gene is sufficient to rescue this bang sensitivity, we utilized a transgene containing the eas+ gene controlled by a heat shock promoter (hs-eas+). Adult mutants with the transgene (genotype eas; hs-eas+/+) were tested daily for bang sensitivity and subjected to daily 37°C heat shocks to induce expression of eas+ (Fig. 1A). We found that bang sensitivity markedly decreased over time when flies received a daily heat shock. After two heat shock treatments (and tested ~22 hours after the last heat shock), mutant flies with the transgene show a significant difference in bang sensitivity compared with control flies (Fig. 1B). Rescue from bang sensitivity was almost fully complete by the fourth and fifth heat shock. In contrast, mutant flies heterozygous for hs-eas+ and not receiving any heat shocks were 100% BS, indicating that the hs-eas+ transgene was not activated to sufficient levels for any behavioral rescue at rearing temperature. To ensure that the heat shocks themselves have no effect on seizure thresholds, such as through the activation of a heat shock response, we also tested eas mutants under the same heat shock regimen but lacking hs-eas+. We found no significant difference in bang sensitivity compared with non heat-shocked eas; hs-eas+/+ control flies, confirming that significant seizure rescue was achieved only with induction of the hs-eas+ transgene (Fig. 1B).
Figure 1.
Induced expression of the eas+ gene in adult flies rescues bang sensitivity. A: Schematic of the bang sensitivity testing and heat shock regimen. Newly eclosed adult flies (0–2 days old) of the appropriate genotype were collected and rested before behavioral testing. On day 0 (number signifies the number of heat shocks received at the time of testing), flies were tested for bang sensitivity by mechanical shock (10-second vortex), and then flies were heat shocked for 2 hours. The protocol was repeated for a total of 5 days. Flies were tested for bang sensitivity approximately 22 hours after each heat shock. B: Populations of adult flies were tested daily for bang sensitivity with and without a 2-hour daily heat shock to induce expression from the transgene. Male flies mutant for eas and containing the hs-eas+ transgene (genotype eas; hs-eas+/+, triangles) showed 43% bang sensitivity (n = 253) after two heat shock treatments, P < 0.0001 on day 2 (χ2 test). After three heat shocks, flies showed 23% bang sensitivity (n = 142), achieving 9% and 12% bang sensitivity upon four and five heat shocks (n = 46, n = 26), respectively. In contrast, heat-shocked control mutants lacking hs-eas+ (genotype eas+/+, circles) showed little change in bang sensitivity, remaining above 90% after five heat shock treatments (n = 44 on day 5). Nonheat-shocked control flies (genotype eas; hs-eas; +/+, squares) showed 100% bang sensitivity throughout the duration of the entire experiment (n = 33 on day 5), P < 0.0001 on day 5 (χ2 test). All error bars represent standard error. C: Activation of the hs-eas+ transgene is not capable of rescuing bang sensitivity in the bang-sensitive mutant bang senseless (parabss1), P = 0.45 on day 5 (χ2 test). D: Rescue of eas bang sensitivity shows stability at least 1 week after discontinuation of heat shocks. Flies of the genotype eas; hs-eas+/+ were given a daily 2-hour heat shock for 3 days and then tested for bang sensitivity 7 days later (corresponding to day 9 on timeline). On day 9, flies were 44% BS (n = 18), in contrast to controls of the same genotype but without any heat shocks, *P < 0.0001 (Fisher’s exact test).
To investigate the possibility that ethanolamine kinase could act as a general seizure suppressor, we tested an additional BS mutant, parabss1, to determine whether seizures could be rescued in a different BS genetic background. The parabss1 mutant represents a dominant missense mutation in the voltage-gated Na+ (NaV) channel gene that alters channel inactivation (Parker et al., 2011b). Mutant parabss1 flies with one copy of the hs-eas+ transgene and given the same heat shock regimen described above showed no changes in bang sensitivity, suggesting that ectopic or overexpression of ethanolamine kinase does not act as a general seizure suppressor in a different seizure-sensitive genetic background (Fig. 1C).
Next, to investigate the stability of rescue in the mutant, we asked whether bang resistance could be maintained over time without further heat shocks (Fig. 1D). We heat shocked eas; hs-eas+/+ flies once per day for 3 consecutive days (three total heat shocks) and then tested them for bang sensitivity 1 week later. Flies tested after receiving a daily heat shock over the course of three days showed partial rescue of bang sensitivity. After 1 week with no heat shocks and left undisturbed 25°C, approximately half of the population still showed rescue from seizure sensitivity compared with controls that were BS. Therefore, an initial burst of eas+ gene expression seems to have a long-lasting effect on the physiology of the fly.
Spatial requirements of ethanolamine kinase
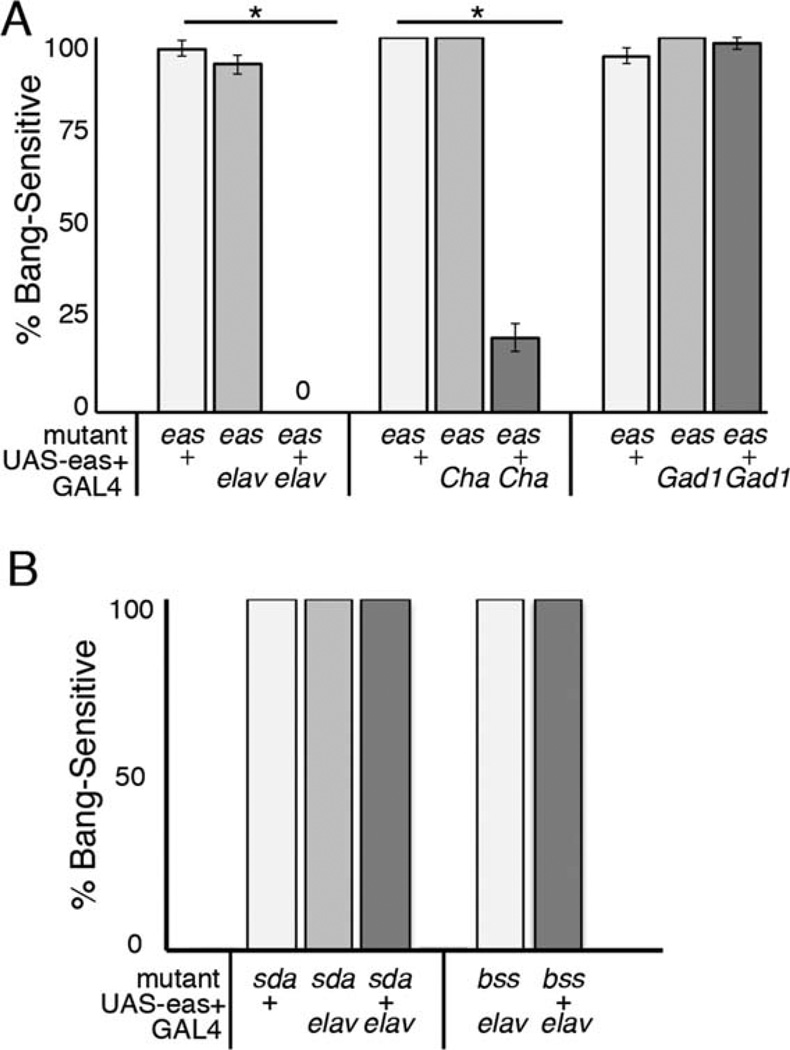
Epilepsy is thought to arise from imbalances in excitation and inhibition, so we separately tested the effects of eas+ expression in cholinergic neurons and GABAergic neurons using the GAL4/UAS system. One interpretation with our use of the GAL4 system is that the neurons targeted by these drivers may represent either distinct excitatory or distinct inhibitory neural circuits. First, we established that expression of the transgene in the entire nervous system was sufficient to rescue from seizures. Expression of eas+ in mutants using the pan-neuronal GAL4 driver elavc155 completely eliminated bang sensitivity, whereas controls were nearly 100% BS, confirming a neural role for the gene (Fig. 2A). Next, to test the contribution of cholinergic neurons in the seizure phenotype, expression of eas+ was achieved using the Cha-GAL4 driver. Adult flies showed 20% bang sensitivity, substantially lower than controls, which showed 100% bang sensitivity (Fig. 2A), indicating partial rescue of the phenotype. In contrast to this, flies expressing of eas+ in GABAergic neurons using the Gad1-GAL4 driver were similar to control eas mutants, indicating no rescue of the phenotype (Fig. 2A). From these results it appears that the effect of eas+ on seizure susceptibility can be attributed primarily to excitatory rather than inhibitory circuits.
Figure 2.
Cell type expression of eas+. A: Cell type specificity of eas+ was achieved using the GAL4/UAS system. Male 1–2-day-old eas flies with pan-neuronal (elavc155-GAL4), cholinergic (Cha-GAL4), or GABAergic (Gad1-GAL4) GAL4 drivers were paired with a UAS-eas+ construct and tested for bang sensitivity by mechanical stimulation. The driver elavc155-GAL4 fully rescued the behavioral phenotype in eas mutants (genotype eas elavc155; UAS-eas+/+), with no flies displaying BS behavior (n = 47), in contrast to the 100% bang sensitivity seen in mutant controls with only the UAS-eas+ or GAL4 construct (n > 68), *P < 0.0001. Expression of eas+ in cholinergic neurons (genotype eas; Cha-GAL4/UAS-eas+) reduced bang sensitivity to 19.8% (n = 116) compared with the bang sensitivity seen in mutant controls (n > 31), P < 0.0001. Flies expressing eas+ in GABAergic neurons (genotype eas; Gad1-GAL4/UAS-eas+) were 95% BS (n = 102), showing no significant difference compared with controls (n > 60), P = 0.07. Error bars represent standard error. Statistical significance was calculated by χ2 test. B: Rescue of bang sensitivity is effective only in the eas mutant background. Expression of eas+ was driven by elavc155-GAL4 in sda and bss mutant background. There was no change in bang sensitivity, with all flies testing 100% BS (n > 22).
Pan-neuronal expression of eas+ using the GAL4 system in other seizure-sensitive mutants failed to achieve any rescue in the BS phenotype (Fig. 2B). Flies mutated for parabss1 or slamdance (sda), a mutation in an amino-peptidase that also causes bang sensitivity (Zhang et al., 2002), showed no difference in sensitivity with or without the UAS-eas+ transgene, further suggesting that the rescue seen is specific only to eas mutants.
Electrophysiological assays
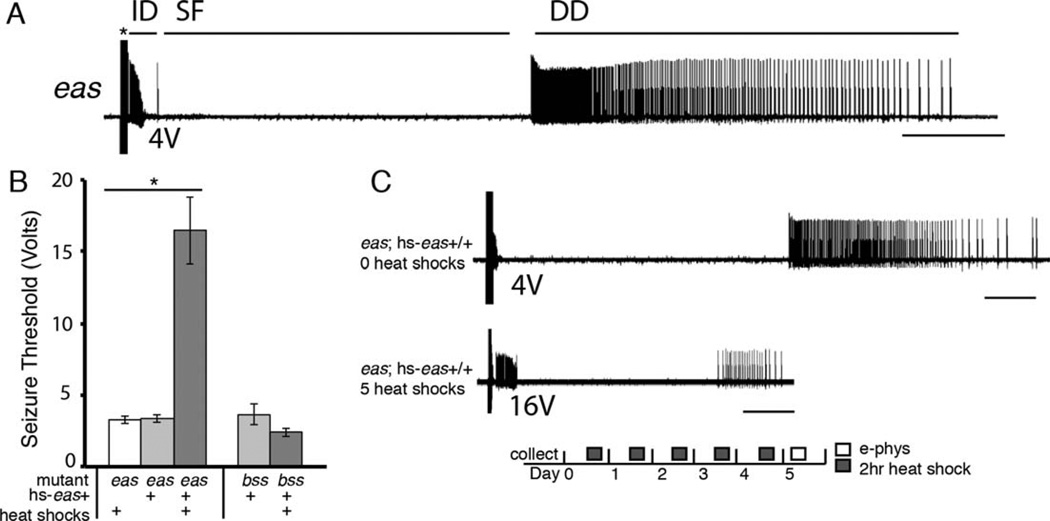
In addition to mechanical stimulation, seizure-like activity in Drosophila can be elicited by high-frequency electrical stimulation (HFS) to the brain. Electrophysiological assays of seizure sensitivity reinforce the conclusions observed from behavioral experiments. A powerful quantitative measurement of a fly’s seizure sensitivity is its seizure threshold, or the minimum voltage of an HFS required to elicit a seizure. Seizure thresholds vary widely between different genotypes: wild-type flies show a threshold of approximately 30 V, whereas eas mutants have a much lower threshold of approximately 3 V (Kuebler et al., 2001). We performed electrophysiological recordings of the DLM of the giant fiber circuit, which acts as a proxy for the state of the entire nervous system during seizure-like activity. Just as in behavioral assays, electrically induced seizure-like activity is stereotypical: beginning with an initial discharge, followed by a period of paralysis characterized by synaptic failure, and ending with a delayed discharge (recovery seizure; Fig. 3A).
Figure 3.
Electrophysiological recordings of seizure activity from the dorsal longitudinal muscle (DLM) of Drosophila. A: Recording of a typical eas mutant seizure. A 4-V high-frequency stimulus (asterisk) causes an initial discharge (ID). The ID is followed by synaptic failure (SF) of the giant fiber circuit, eventually leading to a recovery seizure, or delayed discharge (DD). B: Adult eas flies with the hs-eas+ construct (genotype eas; hs-eas+/+) and given the heat shock regimen for 4 or 5 days showed higher seizure thresholds of 16 ± 2 V (n = 10) compared with sibling flies not receiving any heat shocks, which had thresholds of 3.3 ± 0.2 V (n = 6). Mutants receiving heat shocks but without the transgene show similarly low seizure thresholds of 3.3 ± 0.2 V (n = 6), *P < 0.0001, (ANOVA). Rescue in the bss mutant background was unsuccessful (n = 5 per genotype), P = 0.15 (Student’s t-test). Error bars represent standard error. C: Electrophysiological recordings of the DLM in flies with and without heat shock treatment. An HFS of 4 V triggered a seizure in a fly that had received zero heat shocks (upper trace). A fly that had received the daily 2-hour heat shock regime for 5 days shows a higher seizure threshold; in this case, a 16-V HFS triggers a seizure (lower trace). There is a significant difference in the length of recovery seizures in rescued flies: recovery seizures last for approximately 6 ± 1 seconds (n = 11) for flies receiving heat shocks vs. 25 ± 1 seconds (n = 4) for flies without heat shocks, P < 0.0001 (Student’s t-test). Bar indicates 5 seconds in each trace.
Seizure thresholds were determined for eas mutants heterozygous for hs-eas+ and that received the heat shock regimen depicted in Figure 1A. Flies tested on days 4 and 5 of the regime showed seizure thresholds of approximately 16 V, although controls lacking either the transgene or the heat shock treatments showed much lower seizure thresholds of approximately 4 V (Fig. 3B), indicating that heat shocks and the hs-eas+ transgene must both be present for rescue. Furthermore, we observed a reduction in the duration of recovery seizures in rescued flies. Heat-shocked flies with the transgene showed recovery seizures lasting for approximately 6 seconds compared with controls, whose recovery seizures were approximately 24 seconds in length (P < 0.0001, Student’s t-test). Finally, parabss1 flies heterozygous for the hs-eas+ transgene (parabss1; hs-eas+/+) and given daily heat shock treatments for 5 days showed low seizure thresholds when tested on day 5, indicating no rescue of the seizure-sensitive phenotype and agreeing with behavioral data (Fig. 3C).
To test whether overexpression of eas+ can increase seizure thresholds in wild-type flies, we measured the seizure thresholds in wild-type flies homozygous for hs-eas+ receiving a daily heat shock for 5 days. There was no significant difference in seizure thresholds for flies receiving five heat shocks compared with age-matched controls not receiving heat shocks (28 and 26 V, respectively) indicating that ethanolamine kinase overexpression in a wild-type background does not increase seizure thresholds (P = 0.36, Student’s t-test).
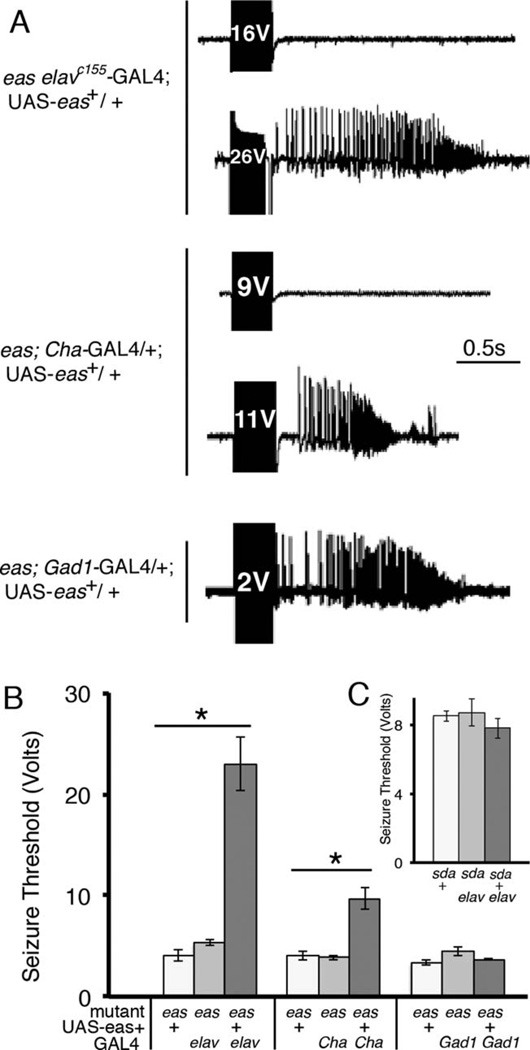
We next examined seizure thresholds in GAL4/UAS rescued flies. Seizure thresholds were highest in flies expressing eas+ pan-neuronally, which showed thresholds of approximately 23 V, compared with controls, which showed lower thresholds (Fig. 4A,B). Flies expressing eas+ in cholinergic neurons showed seizure thresholds of about 10 V, indicating partial rescue of the seizure threshold compared with pan-neuronal expression (Fig. 4A,B). In agreement with the behavioral results is the finding that flies expressing eas+ in GABAergic neurons showed seizure similar thresholds to those of controls, with all having seizures at low voltages (Fig. 4A,B). Overall, these results verify our previous behavioral data, indicating that a portion of the phenotypic rescue can occur with eas+ expression limited to Cha-GAL4 cholinergic neurons, suggesting that the eas seizure defect results from excess excitation rather than insufficient inhibition.
Figure 4.
Electrophysiological recordings and seizure thresholds in flies expressing eas+ in subsets of the nervous system. A: Recordings from the DLM muscle in 1–2-day-old male eas mutant flies expressing eas+ in different neuronal populations using the GAL4/UAS system. A fly expressing eas+ under control of the GAL4 driver elavc155 (genotype eas elavc155-GAL4; UAS-eas+) shows a high seizure threshold in the wild-type range (top two traces). Additionally, a fly expressing eas+ in cholinergic neurons has a seizure threshold more than double compared with controls: a seizure is triggered at 11 V (genotype eas; UAS-eas+/Cha-GAL4, third and fourth traces). In contrast, a fly expressing eas+ in GABAergic neurons (genotype eas; UAS-eas+/Gad1-GAL4, fifth trace) shows low seizure thresholds similar to controls, indicating mutant seizure sensitivity. B: Seizure thresholds were determined for different genotypes. Pan-neuronal expression using the elavc155-GAL4 driver provided the strongest rescue, with seizure thresholds reaching 23 ± 3 V (n = 7), similar to wild-type thresholds. Controls in the eas mutant background containing either UAS-eas+ or the GAL4 showed low seizure thresholds of about 4.5 V (n > 7), *P < 0.0001 (ANOVA). The seizure threshold for flies expressing eas+ in cholinergic neurons using the Cha-GAL4 driver was 9.7 ± 1 V (n = 9), higher than that of control mutant flies lacking the GAL4 transgene or lacking the UAS-eas+ construct, which showed seizure thresholds of 4 ± 0.4 V (n = 7) and 3.8 ± 0.2 V (n = 8), respectively, P < 0.0001 (ANOVA). There was no significant difference in seizure thresholds for flies expressing eas+ in GABAergic neurons using the Gad1-GAL4 driver compared with controls (n > 5), P = 0.14 (ANOVA). C: Seizure thresholds do not increase in the sda mutant background. Pan-neuronal expression of eas+ in the sda mutant background did not change seizure thresholds significantly (n > 5), P = 0.50 (ANOVA). Error bars represent standard error.
DISCUSSION
Phospholipids are an underappreciated but fundamentally important class of biomolecules that form the structure of neuronal membranes and synapses and underlie signaling in many transduction pathways (Weber et al., 2003). The eas+ gene encodes ethanolamine kinase, required for synthesis of PtdEtn via the CDP-ethanolamine branch of the Kennedy pathway (Gibellini and Smith, 2010). In contrast to the case in mammals, PtdEtn is the most abundant phospholipid in Drosophila, accounting for more than half of the total plasma membrane composition (Jones et al., 1992). Analysis of ion mobility mass spectrophotometry reveals only ~40 of 1,200 phospholipid signals showing significant changes in eas brains compared with wild type (Kliman et al., 2010). In addition to causing a slight increase in certain phospholipid classes, such as PtdCho, the eas mutation changes the concentrations of phospholipid species within a given phosopholipid class. For example, five PtdEtn species that show an increase in the mutant brain tissue correspond to species produced primarily by synthesis pathways other than the Kennedy pathway, whereas three PtdEtn species that show a decrease in the mutant are synthesized primarily from the Kennedy pathway (Bleijerveld et al., 2007; Kliman et al., 2010).
Ethanolamine kinase is known to play a prominent role in nervous system development; eas null mutants fail to develop mushroom body α/β lobes properly. The loss of these structures appears to be a result of a slower pace of neuroblast cell division that is rescued by eas+ expression during late larval development (Pascual et al., 2005). Because of this, an expectation was that defects in brain development might result in the kinds of structural changes that would underlie seizure sensitivity. Surprisingly, this was not the case. The results presented here show that any possible structural changes resulting from lack of eas+ in development have a minimal effect. Therefore, in general, the observed structural changes made to the brain are not indicative of the type of mechanism leading to seizure sensitivity.
We were able to rescue adult flies from seizure sensitivity without regard to the effects of expression level of eas+ or problems with cell type specificity, suggesting that the exact dosage of ethanolamine kinase is fairly unimportant for overall physiology of the fly. Behavior appeared completely normal upon induction of the eas+ gene, indicating few, if any, detrimental effects on induced global expression.
We used the GAL4/UAS system to express a wild-type version of the eas+ gene. Although the GAL4 lines used in this study might not perfectly target the cholinergic and GABAergic neural populations, they largely represent those neural populations and allow general comparisons to be made between them. Our results suggest that eas+ is critical for stabilizing excitatory processes, without a concomitant effect on inhibition. In a model for seizure susceptibility initially proposed by Kuebler et al. (2001), seizure susceptible mutants require stimulation of fewer neurons in the “neuron pool” than wild-type flies to initiate a seizure. This susceptibility could be due to an increased ratio of excitatory to inhibitory neurons in the neuronal pool or could be the result of specific changes to the strength of excitation and inhibition in a balanced neuronal pool. The results presented here show that seizures can be rescued postdevelopmentally, so seizure susceptibility in the eas mutant is not due to a change in the ratio of excitatory to inhibitory neurons; otherwise, rescue would have been achieved during development. Instead, our results suggest that there are changes in the contribution of excitatory processes within the neuronal pool that result in seizure sensitivity.
Defects in metabolism can manifest themselves in a cell-type-specific manner, causing epilepsy. For example, mutations in the mouse gene alkaline phosphatase, involved in vitamin B6 metabolism, affects seizure sensitivity via GABAergic neurons. Mice mutated for this important metabolic gene show reduced levels of GABA, causing seizure phenotypes that lead to lethality (Waymire et al., 1995). However, low levels of GABA and lethality could be rescued with supplementation of pyridoxil, a molecule within the affected pathway. In Drosophila, acetylcholine is the primary excitatory neurotransmitter in the CNS. Cholinergic neurons may be more susceptible to the effects of the eas mutation than other neuron types, because choline is used as a precursor for neurotransmitter synthesis as well as an initial substrate for the synthesis of PtdCho (Wurtman, 1992).
Alterations to phospholipid composition in the membrane could enhance neural excitability in multiple ways. Proper phospholipid composition has been shown to be important for maintaining the proper function and orientation of transmembrane proteins, and PtdEtn has been shown to act as a molecular lipo-chaperone within the plasma membrane to assist in protein folding (Bogdanov et al., 1996, 1999; Bogdanov and Dowhan, 1998). Additionally, some studies suggest that the biochemical properties of PtdEtn can create extra rigidity within the membrane, which could promote the fusion of membranes and vesicles (Birner et al., 2001). The defect could also interfere with membranes of organelles, such as the mitochondria (Fergestad et al., 2008). However, our results suggest that there may be cell-type-specific requirements for different phospholipids, contributing to the complexity of analysis.
Other types of disorders seem to be linked to phospholipid defects, suggesting that phospholipid homeostasis is a critical component of human health. There is evidence for lowered levels of PtdEtn in the brains of Alzheimer’s disease patients (Wells et al., 1995), and other neurological disorders such as attention deficit-hyperactivity disorder, autism, schizophrenia, and dyslexia have been theorized to involve defects in phospholipid metabolism (Brown and Austin, 2011; Bennett and Horrobin, 2000). Some of these neurological disorders, including epilepsy, can occur together in the same patient, so it is intriguing to consider whether some disorders currently thought to originate from developmental defects could actually represent defects in phospholipid homeostasis.
Unlike many other diseases, current epilepsy drugs only target the symptoms of epilepsy rather than correcting the specific deficit that causes seizure susceptibility. We show that certain types of epilepsy have the potential to be eliminated by directly addressing the molecular deficit and that there may be flexibility in the time window for treatment, suggesting gene therapy as a potential future cure for certain epilepsy-related disorders.
ACKNOWLEDGMENTS
We thank Zeid M. Rusan and other members of the Tanouye Laboratory for helpful discussions throughout the project.
Grant sponsor: McKnight Foundation (to M.A.T.); Grant sponsor: National Institutes of Health; grant number: NS31231 (to M.A.T.).
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest.
ROLE OF AUTHORS
Both authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: JRK, MAT. Acquisition of data: JRK. Analysis and interpretation of data: JRK, MAT. Drafting of the manuscript: JRK, MAT. Statistical analysis: JRK. Obtained funding: MAT.
LITERATURE CITED
- 1.Bennett CN, Horrobin DF. Gene targets related to phospholipid and fatty acid metabolism in schizophrenia and other psychiatric disorders: an update. Prostaglandins Leukot Essent Fatty Acids. 2000;63:47–59. doi: 10.1054/plef.2000.0191. [DOI] [PubMed] [Google Scholar]
- 2.Birner R, Bürgermeister M, Schneiter R, Daum G. Roles of phosphatidylethanolamine and of its several biosynthetic pathways in Saccharomyces cerevisiae. Mol Biol Cell. 2001;12:997–1007. doi: 10.1091/mbc.12.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleijerveld OB, Brouwers JFHM, Vaandrager AB, Helms JB, Houweling M. The CDP-ethanolamine pathway and phosphatidylserine decarboxylation generate different phosphatidylethanolamine molecular species. J Biol Chem. 2007;282:28362–28372. doi: 10.1074/jbc.M703786200. [DOI] [PubMed] [Google Scholar]
- 4.Bogdanov M, Dowhan W. Phospholipid-assisted protein folding: phosphatidylethanolamine is required at a late step of the conformational maturation of the polytopic membrane protein lactose permease. EMBO J. 1998;17:5255–5264. doi: 10.1093/emboj/17.18.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogdanov M, Sun J, Kaback HR, Dowhan W. A phospholipid acts as a chaperone in assembly of a membrane transport protein. J Biol Chem. 1996;271:11615–11618. doi: 10.1074/jbc.271.20.11615. [DOI] [PubMed] [Google Scholar]
- 6.Bogdanov M, Umeda M, Dowhan W. Phospholipid-assisted refolding of an integral membrane protein. Minimum structural features for phosphatidylethanolamine to act as a molecular chaperone. J Biol Chem. 1999;274:12339–12345. doi: 10.1074/jbc.274.18.12339. [DOI] [PubMed] [Google Scholar]
- 7.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 8.Brown CM, Austin DW. Autistic disorder and phospholipids—a review. Prostaglandins Leukot Essent Fatty Acids. 2011;84:25–30. doi: 10.1016/j.plefa.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Claes L, Del-Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68:1327–1332. doi: 10.1086/320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fergestad T, Olson L, Patel KP, Miller R, Palladino MJ, Ganetzky B. Neuropathology in Drosophila mutants with increased seizure susceptibility. Genetics. 2008;178:947–956. doi: 10.1534/genetics.107.082115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibellini F, Smith TK. The Kennedy pathway—de novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life. 2010;62:414–428. doi: 10.1002/iub.337. [DOI] [PubMed] [Google Scholar]
- 12.Jones HE, Harwood JL, Bowen ID, Griffiths G. Lipid composition of subcellular membranes from larvae and prepupae of Drosophila melanogaster. Lipids. 1992;27:984–987. doi: 10.1007/BF02535576. [DOI] [PubMed] [Google Scholar]
- 13.Kliman M, Vijayakrishnan N, Wang L, Tapp JT, Broadie K, McLean JA. Structural mass spectrometry analysis of lipid changes in a Drosophila epilepsy model brain. Mol Biosyst. 2010;6:958. doi: 10.1039/b927494d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuebler D, Tanouye MA. Modifications of seizure susceptibility in Drosophila. J Neurophysiol. 2000;83:998–1009. doi: 10.1152/jn.2000.83.2.998. [DOI] [PubMed] [Google Scholar]
- 15.Kuebler D, Zhang H, Ren X, Tanouye MA. Genetic suppression of seizure susceptibility in Drosophila. J Neurophysiol. 2001;86:1211–1225. doi: 10.1152/jn.2001.86.3.1211. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Wu CF. Genetic modifications of seizure susceptibility and expression by altered excitability in Drosophila Na+ and K+ channel mutants. J Neurophysiol. 2006;96:2465–2478. doi: 10.1152/jn.00499.2006. [DOI] [PubMed] [Google Scholar]
- 17.Parker L, Howlett IC, Rusan ZM, Tanouye MA. Seizure and epilepsy: studies of seizure disorders in Drosophila. Int Rev Neurobiol. 2011a;99:1–21. doi: 10.1016/B978-0-12-387003-2.00001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker L, Padilla M, Du Y, Dong K, Tanouye MA. Drosophila as a model for epilepsy: bss is a gain-of-function mutation in the para sodium channel gene that leads to seizures. Genetics. 2011b;187:523–534. doi: 10.1534/genetics.110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pascual A, Chaminade M, Preat T. Ethanolamine kinase controls neuroblast divisions in Drosophila mushroom bodies. Dev Biol. 2005;280:177–186. doi: 10.1016/j.ydbio.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Pavlidis P, Ramaswami M, Tanouye MA. The Drosophila easily shocked gene: a mutation in a phospholipid synthetic pathway causes seizure, neuronal failure, and paralysis. Cell. 1994;79:23–33. doi: 10.1016/0092-8674(94)90397-2. [DOI] [PubMed] [Google Scholar]
- 21.Song J, Tanouye MA. Seizure suppression by shakB2, a gap junction mutation in Drosophila. J Neurophysiol. 2006;95:627–635. doi: 10.1152/jn.01059.2004. [DOI] [PubMed] [Google Scholar]
- 22.Song J, Parker L, Hormozi L, Tanouye MA. DNA topoisomerase I inhibitors ameliorate seizure-like behaviors and paralysis in a Drosophila model of epilepsy. Neuroscience. 2008;156:722–728. doi: 10.1016/j.neuroscience.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun L, Gilligan J, Staber C, Schutte RJ, Nguyen V, O’Dowd DK, Reenan R. A knock-in model of human epilepsy in Drosophila reveals a novel cellular mechanism associated with heat-induced seizure. J Neurosci. 2012;32:14145–14155. doi: 10.1523/JNEUROSCI.2932-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waymire KG, Mahuren JD, Jaje JM, Guilarte TR, Coburn SP, MacGregor GR. Mice lacking tissue nonspecific alkaline phosphatase die from seizures due to defective metabolism of vitamin B-6. Nat Genet. 1995;11:45–51. doi: 10.1038/ng0995-45. [DOI] [PubMed] [Google Scholar]
- 25.Weber U, Eroglu C, Mlodzik M. Phospholipid membrane composition affects EGF receptor and Notch signaling through effects on endocytosis during Drosophila development. Dev Cell. 2003;5:559–570. doi: 10.1016/s1534-5807(03)00273-9. [DOI] [PubMed] [Google Scholar]
- 26.Wells K, Farooqui AA, Liss L, Horrocks LA. Neural membrane phospholipids in Alzheimer disease. Neurochem Res. 1995;20:1329–1333. doi: 10.1007/BF00992508. [DOI] [PubMed] [Google Scholar]
- 27.Wurtman RJ. Choline metabolism as a basis for the selective vulnerability of cholinergic neurons. Trends Neurosci. 1992;15:117–122. doi: 10.1016/0166-2236(92)90351-8. [DOI] [PubMed] [Google Scholar]
- 28.Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 2006;9:1142–1149. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Tan J, Reynolds E, Kuebler D, Faulhaber S, Tanouye M. The Drosophila slamdance gene: a mutation in an aminopeptidase can cause seizure, paralysis and neuronal failure. Genetics. 2002;162:1283–1299. doi: 10.1093/genetics/162.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y-D, Lee S, Jin Z, Wright M, Smith SEP, Anderson MP. Arrested maturation of excitatory synapses in autosomal dominant lateral temporal lobe epilepsy. Nat Med. 2009;15:1208–1214. doi: 10.1038/nm.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]