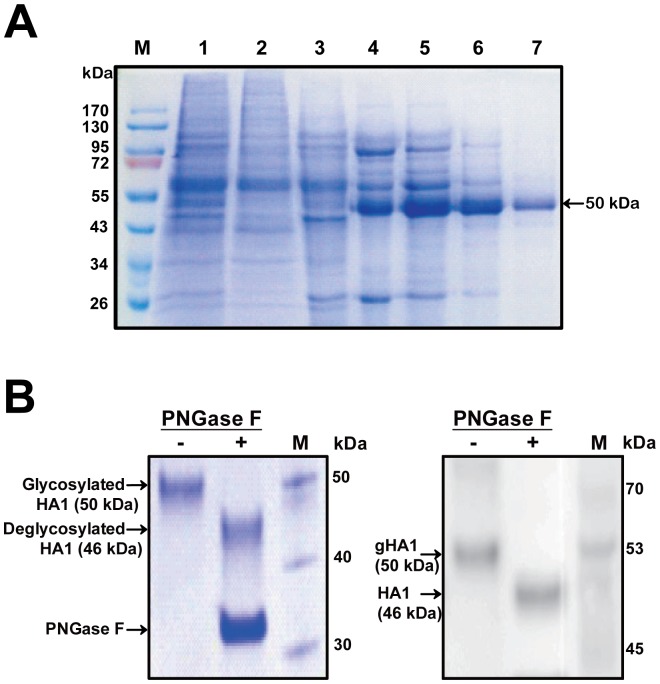
Figure 1. Purification of the gHA1 from insect cells.
(A) SDS-PAGE analysis of AIV HA protein expressed in a baculovirus/insect cell system. His-tagged recombinant hemagglutinin protein (gHA1) was purified using Ni-NTA affinity chromatography and gel filtration. Whole supernatant of insect cell (TriEx Sf9) culture was first loaded onto a Ni-NTA affinity chromatography column (Lane 1). Lanes 2 and 3 are the flow-through and washing eluants through the Ni-NTA affinity column, respectively. Lanes 4 to 6 are collected fractions by imidazole elution. Lane 7 is purified gHA1 after gel filtration chromatography (indicated with an arrow). (B) Cleavage of glycans from the purified gHA1. The purified gHA1 (3 µg) was incubated with (+) or without (–) PNGase F, and the reaction mixture was resolved with 12% SDS-PAGE (left panel). HA was probed with anti-HA polyclonal antibody (right panel). The migration of PNGase F treated (+) and untreated (–) recombinant HA1 is indicated with arrows.