Abstract
Background
DSM-IV specifies a hierarchal diagnostic structure such that an ODD diagnosis is applied only if criteria are not met for CD. Genetic studies of ODD and CD support a combination of shared genetic and environmental influences, but largely ignore the imposed diagnostic structure.
Methods
We examined whether ODD and CD share an underlying etiology while accounting for DSM-IV diagnostic specifications. Data from 1446 female twin pairs, aged 11–19, were fitted to two-stage models adhering to the DSM-IV diagnostic hierarchy.
Results
Models suggested that DSM-IV ODD-CD covariation is attributed largely to shared genetic influences.
Conclusions
This is the first study, to our knowledge, to examine genetic and environmental overlap among these disorders while maintaining DSM-IV hierarchical structure. Findings reflect primarily shared genetic influences and specific (i.e., uncorrelated) shared/familial environmental effects on these DSM-IV defined behaviors. These results have implications for how best to define CD and ODD for future genetically-informed analyses.
Keywords: adolescence, conduct disorder, genetics, oppositional defiant disorder, twins
Introduction
Oppositional Defiant Disorder (ODD) and Conduct Disorder (CD) represent two of the leading diagnoses for youth referred to psychiatric care (Loeber et al., 2000). Further, ODD and CD are associated with later substance use problems (White et al., 2001), multiple mood and anxiety disorders (Nock et al., 2007), and antisocial personality disorder (Kim-Cohen et al., 2003). The Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; APA, 1994) defines ODD as a “pattern of negativistic, hostile, and defiant behavior” toward authority figures continuing for at least 6 months and resulting in significant impairment in functioning. Full DSM-IV ODD diagnosis requires the presence of at least four of eight discrete symptoms (Table 1). CD is characterized by the violation of “the basic rights of others… or societal norms or rules” and significant impairment in functioning occurring in an individual under the age of 18. Full DSM-IV CD diagnosis requires at least three of 15 symptoms (Table 1) within the past year and at least one symptom within the past three months. ODD and CD share unifying themes of problem behavior in excess of developmental and societal norms, and some CD symptoms appear to represent a more extreme version of ODD symptoms. Despite these unifying themes, the DSM-IV considers CD and ODD distinct diagnostic entities but specifies a hierarchal structure such that a diagnosis of ODD may be applied only if criteria are not met for CD. This is in contrast to the International Classification of Diseases, Version 10 (ICD-10; WHO, 1992) in which ODD is treated as a subtype of CD (for a more complete examination of DSM-IV and ICD-10 diagnoses of CD and ODD, see (Rowe et al., 2005).
Table 1.
DSM-IV items for Oppositional Defiant Disorder (ODD) and Conduct Disorder (CD)
| ODD items | CD items |
|---|---|
| Often loses temper | Often bullies, threatens or intimidates others |
| Often argues with adults | Often initiates physical fights |
| Often actively defies or refuses to comply with adults’ requests or rules | Has used a weapon that can cause serious physical harm to others |
| Often deliberately annoys people | Has been physically cruel to people |
| Often blames others for his or her mistakes or misbehavior | Has been physically cruel to animals |
| Is often touchy or easily annoyed by others | Has stolen while confronting a victim (e.g., mugging, armed robbery) |
| Is often angry or resentful | Has forced someone into sexual activity |
| Is often spiteful or vindictive | Has deliberately engaged in fire setting |
| Has deliberately destroyed others’ property | |
| Has broken into someone else’s house, building, or car | |
| Often lies to obtain goods or favors or to avoid obligations (i.e., ‘cons’ others) | |
| Has stolen items of nontrivial value without confrontation (e.g., forgery) | |
| Often stays out at night despite parental prohibitions, beginning before age 13 | |
| Has run away from home overnight | |
| Often truant from school |
The DSM-IV conceptualization and specification of ODD and CD lead to an important question: Are DSM-IV ODD and CD etiologically distinct or are they manifestations of an underlying shared liability? We can address this through genetically-informed designs, which can aid in clarifying the pathogenesis of these behaviors by disentangling sources of covariance while simultaneously allowing the DSM-IV hierarchical structure. Prior studies have suggested a shared liability, suggesting that etiological studies can safely combine CD and ODD symptoms. However, most studies to date have either not used DSM-IV criteria due to timing of data collection or failed to accurately model the DSM-IV hierarchical structure specified for these two disorders. Thus, in this report, we address the questions: (i) What is the etiological structure of the covariation between DSM-IV CD and ODD? (ii) Does the etiological structure support the practice of combining CD and ODD when the DSM-IV diagnostic hierarchy is accurately reflected?
Are ODD and CD etiologically distinct?
Genetically-informative designs are ideal for the exploration of whether disorders share the same etiology because biometric models can be used to directly test the degree to which symptoms of each disorder, as well as their comorbidity, are explained by common genetic and environmental influences (Rhee et al., 2008). Univariate models of CD and ODD suggest that genetic influences are important for each diagnosis (Ehringer et al., 2006) and that shared environment plays an important role in CD (e.g. Knopik et al., 2009). For the current report, we are primarily interested, however, in whether these two disorders share genetic or environmental influences. More specifically, can, or should, we treat them separately or can we combine them for future genetically-informed investigations?
Most studies looking at ODD and CD have created an ODD/CD phenotype by combining ODD/CD symptoms (Nadder et al., 2002, Silberg et al., 1996) and thus making the assumption that these disorders can be combined from an etiological perspective. These investigations have generally provided support for the importance of genetic influences on the ODD/CD phenotype and, because it is typically modeled in the same analyses, a genetic correlation with attention-deficit hyperactivity disorder (ADHD), while the role of shared environment was negligible (Nadder et al., 2002, Silberg et al., 1996). Other investigators, however, have reported the importance of shared environmental effects to the relationship between the ODD/CD phenotype and ADHD (Burt et al., 2003, Burt et al., 2001).
There are also a handful of studies that consider the etiology of the covariation between ODD and CD, when modeled as separate behaviors. For example, an investigation that modeled ODD and CD separately (as part of a larger model including ADHD), found support for shared genetic influence across the three phenotypes in a sample of 14 year old twins (Dick et al., 2005). Eaves and colleagues also explored the shared genetic influences on DSM-III ADHD, ODD, and CD in 8–18 year old twins, with findings suggesting that ODD and CD were more strongly genetically correlated than either disorder was with ADHD (Eaves et al., 2000).
In one of the only studies to date that considered ODD and CD separately using DSM-IV symptom criteria, Tuvblad and colleagues (Tuvblad et al., 2009) examined shared genetic and environmental influences on DSM-IV symptoms of ADHD, ODD, and CD in 9–10 year old twins; however, the DSM-IV hierarchical structure was not modeled. Findings supported a latent externalizing behavior factor underlying covariance among ADHD, ODD, and CD, with most of the variance (57%) attributable to genetic influences and 19% associated with non-shared environment. Shared environment did not contribute to the variance of the latent externalizing factor.
There are many risk factors that might plausibly be expected to influence both ODD and CD (e.g., family history of antisocial personality disorder or substance dependence). However, it is also highly likely that there are genetic influences or environmental influences that influence the aggressive behaviors of CD but have no influences on ODD (e.g., (Kendler et al., 2013). Dissecting common versus specific influences on DSM-IV ODD versus CD is important from many perspectives. For the purposes of gene-mapping studies of disruptive behavior, for example, it would be important to know whether genetic influences observed for ODD reflect genetic influences on differences among individuals with risk for ODD, or merely genetic influences on CD that are shared and might be explained by personality or other heritable risk factors. For the purposes of prevention research, it would be important to understand whether genotype × environment interaction effects are arising through influences on genetic effects associated with ODD that may also influence CD, versus genetic effects that specifically influence CD. For example, results might help to inform why not all children with ODD progress to CD (Rowe et al., 2005) or why ODD has been associated with higher levels of comorbid psychopathology than CD (Maughan et al., 2004).
Current Study
The majority of extant studies examining genetic and environmental overlap between ODD and CD have (a) included ADHD, and (b) used DSM-III or other diagnostic criteria or, if using DSM-IV, did not model the hierarchical structure. Given the common research practice of combining CD and ODD into one outcome and the clear hierarchical nature of DSM-IV criteria for these two disorders, it is important to empirically determine how best to handle these constructs in future genetically-informative research. Thus, in a sample of female twins, we examine the etiological structure of DSM-IV ODD and CD without ADHD and determine whether, in the absence of ADHD, CD and ODD are etiologically distinct or share a common underlying liability. We used a two-stage modeling strategy (Heath et al., 2002b) to examine genetic and environmental influences on both outcomes as well as the covariance between them, while accurately reflecting the DSM-IV imposed structure for these disorders. Thus, we examine the overlap between these disruptive behavior disorders while closely approximating the diagnostic process undertaken by clinicians.
Methods
Participants
Data were from the Missouri Adolescent Female Twin Study (MOAFTS), a sample of female adolescent twin pairs and their parents. MOAFTS is a longitudinal study of the development of alcohol problems and associated psychopathology in females (Heath et al., 2002a, Waldron et al., 2012). All twin pairs born between July 1, 1975 and June 30, 1985 in Missouri were identified from birth records. Ascertainment of families occurred from 1995–1998. After exclusion of families with no maternal diagnostic interview and missing data, 1446 twin pairs (~65% of identified families; for details on nonparticipation see (Heath et al., 2002a) with complete data on all variables were included in the present analysis [831 monozygotic (MZ) pairs, 615 dizygotic (DZ) pairs]. 13% of the sample classified themselves as minority and almost exclusively as African-American, reflecting the minority composition of the Missouri population. Self-reported maternal education levels included 9.8% ‘without high school diploma,’ 39.5% ‘high school diploma without any college education,’ 29.2% ‘some college education,’ and 21.4% ‘degree from 4-year college or more.’
Measures
A brief initial parental interview about zygosity (Nichols and Bilbro, 1966) was conducted. Comprehensive structured diagnostic telephone interviews were scheduled with parents and twin pairs. Verbal consent was obtained from all participants prior to participation, as well as parental consent for the participation of their minor children. Minor children were also required to provide verbal assent. The Institutional Review Board at Washington University, St Louis approved all procedures.
Assessment
The parent interview was a modified version of the Semi-Structured Assessment of the Genetics of Alcoholism (SSAGA; Bucholz et al., 1994), which is a comprehensive interview that assesses physical, psychological, social, and psychiatric manifestations of alcohol abuse/dependence and related psychiatric disorders in adults. Modifications were made to the SSAGA to incorporate DSM-IV (APA, 1994) criteria as well as to adapt it for telephone use (see (Bucholz et al., 1994, Hesselbrock et al., 1999) for SSAGA reliability and validity data). In this interview, parents (typically mothers) were asked to report about behaviors in the twins, including symptoms of ODD. Parent report, as well as twin self-report, was based on the Diagnostic Interview for Children and Adolescents (DICA) (Reich, 2000) and the C-SSAGA (SSAGA-Child Version) adapted for telephone use.
Child Conduct Disorder and Oppositional Defiant Disorder
Lifetime child CD and ODD was based on the DICA (Reich, 2000). CD was based on twin self-report of the fifteen DSM-IV items endorsed and ODD was based on maternal report of the eight DSM-IV items endorsed (Table 1). The use of adolescent ratings for CD and maternal ratings for ODD is supported by findings demonstrating that, although prevalence estimates do not differ for ODD or CD between maternal and adolescent ratings, adolescent ratings of CD result in stronger agreement with lifetime diagnosis from a clinical interview and might better capture true behavior as parents are sometimes unaware of CD-consistent behaviors (Rothen et al., 2009). Maternal ratings of ODD, similar to maternal ratings of ADHD (Biederman et al., 2007), meaningfully capture ODD symptomatology due to the fact that ODD behavior is directed towards authority figures and is typically more noticeable at home.
Data preparation
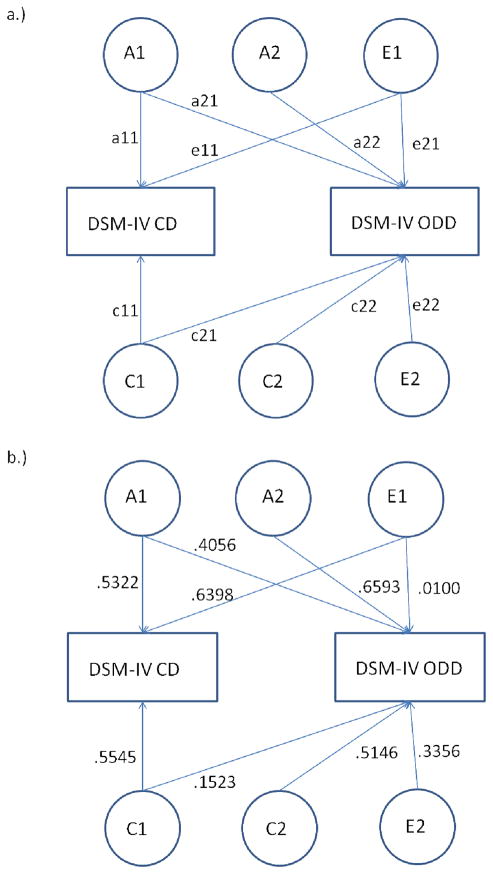
Using a two-stage genetic model, we moved beyond an either/or diagnosis for both CD and ODD, which would have resulted in a binary measure for each disorder. Prior simulations for this two-stage strategy have indicated increased statistical power when outcomes are defined using multiple categories. More specifically, in the two-stage model, it is ideal for the first variable (i.e., CD, Figure 1a), to have at least three categories, at least two of which include individuals who can be assessed on the second variable (i.e., ODD). It is also preferable to define the second variable as a quantitative or multiple-category variable (Heath et al., 2002b). In two-stage models using binary measures, the variance components will not be seriously biased; however, a serious bias may arise for estimates of the genetic and environmental correlations between outcomes (Heath et al., 2002b). These problems can be reduced if several ordered categories can be defined for outcomes.
Figure 1.
(a) Full two-stage model specification (shown for one twin only) and (b) parameter estimates from the full model (Model 1, Table 4). For our two-stage modeling approach and per the DSM-IV hierarchy, ODD was set to missing if an individual endorsed 3+ CD symptoms. A=Additive genetic, C=Shared Environment, E=nonshared Environment
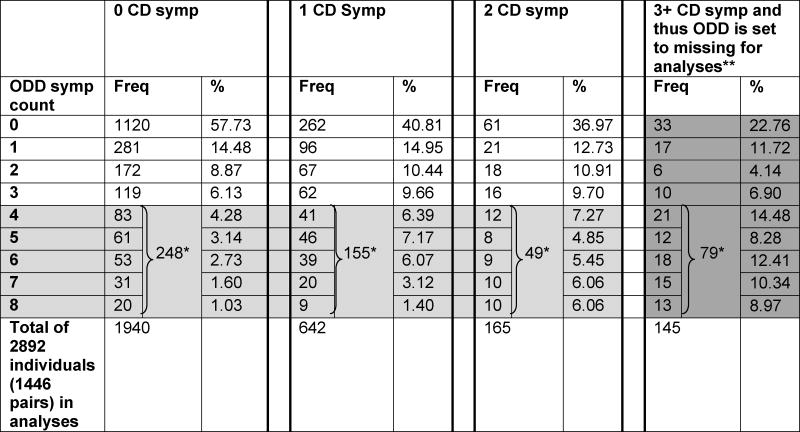
Thus, in order to maximize power and capitalize on the empirical patterns in our data, CD was defined using multiple categories: 0 symptoms, 1 symptom, 2 symptoms, or 3+ symptoms. ODD was similarly defined using multiple categories: missing, 0 symptoms, 1 symptom, 2 symptoms, 3 symptoms, or 4+ symptoms. Only in the case where an individual endorsed 3+ CD symptoms was their ODD value was set to missing. This data preparation step was done prior to the structural two-stage modeling and was done in order to align with the DSM-IV diagnostic structure. It is important to note that by modeling the data in this way, we exclude only ODD data (i.e., set only ODD to missing) in 145 cases, while leaving the CD value in these 145 cases in the model to contribute to the estimation of variance components for CD. The ODD profiles of these excluded cases as well as for all other categories of CD are in Table 2. Thus, the model jointly analyzes DSM-IV CD and ODD using all information about CD and, per DSM-IV, ODD information from all individuals who do not meet prior criteria for CD. Analysis of our DSM-IV CD and ODD variables, based on the Maximum Likelihood estimation of the polychoric correlation (see Olsson, 1979), indicated no deviation from bivariate normality (p=0.5517).
Table 2.
Two-way (CD and ODD) Contingency Table and Profiles of ODD symptom counts for each category of CD. The light gray shaded cells represent the 4+ ODD symptom category and are shown broken down by number of symptoms as well as the sum total. The dark gray shaded cells are not observable (i.e., represent structural missing data) because those with 3+ CD symptoms cannot be characterized on the ODD dimension.
|
Light gray shaded area is the 4+ ODD symptom category for our model fitting.
Dark gray shaded area explicitly shows the part of the table that is unobservable according to DSM-IV and thus may be considered to summarize structural missing data, because those with a CD diagnosis cannot be characterized on the ODD dimension.
Data-analysis
Genetic model-fitting
In order to determine the extent of genetic and environmental influences on risk of DSM-IV CD and ODD, structural equation models were fitted to the data using Mx (Neale et al., 2003). In genetic twin analyses, models are tested that partition variance in an outcome into genetic [additive (A) and non-additive (D)] and environmental [shared (C) and nonshared (E)] components. Additive genetic influences (A) describe the effect of multiple genes that exert influence in a linear or additive fashion. In general, non-additive genetic effects describe interactive effects of different alleles and include genetic dominance (within locus interaction) and epistasis (across locus interaction); however, most twin studies interpret non-additive effects as genetic dominance (Rettew et al., 2008). Shared environmental effects (C) are those influences that make members of a family more similar to one another. Nonshared environmental effects (E) make members of twin pairs different. E also includes measurement error. We denote: a2 for the proportion of total variance due to additive genetic effects, d2 for non-additivity, c2 for shared environment, and e2 for nonshared environmental contributions.
Genetic modeling takes advantage of the differing degrees of genetic relatedness among MZ versus DZ twin pairs. MZ twins share all additive and non-additive genetic effects, while DZ pairs share, on average, 50% of additive and 25% of non-additive genetic effects. Shared environmental effects are assumed to correlate 1.0 between members of both MZ and DZ pairs. Consequently, the phenotypic correlation between MZ twin pairs is rMZ = a2 + d2 + c2 and the phenotypic correlation between members of DZ pairs is rDZ = .5a2 + .25d2 + c2. Examining the pattern of MZ and DZ correlations can provide guidance on model fitting strategy, such that (a) .5rMZ = rDZ, suggests additive genetic influences; (b) .5rMZ < rDZ, suggests both additive genetic and shared environmental influences; or (c) .5rMZ > rDZ, suggests additive and non-additive genetic influences.
The pattern of univariate and cross-twin cross-trait polychoric correlations (Table 3) suggest that for CD, ODD, and comorbid CD-ODD, additive (rather than both additive and dominant) genetic factors influence both the variance and covariance. The pattern also suggests important shared environmental influences for the variance of both outcomes, but not necessarily for the comorbidity between CD and ODD. Thus, our model fitting includes A, C, and E components.
Table 3.
Polychoric and cross-twin correlations between DSM-IV defined CD and ODD categories used in the two stage model.
| MZ Females | ||
| CD | ODD | |
| CD | .60 (.53–.64) | |
| ODD | .27 (.16–.37) | .89 (.86–.91) |
| DZ Females | ||
| CD | ODD | |
| CD | .44 (.34–.53 | |
| ODD | .14 (.02–.25) | .57 (.48–.64) |
Two-stage genetic model
In order to examine whether DSM-IV CD and ODD are etiologically distinct or exhibit shared liability, a two-stage model (Figure 1a) was fit to the data. This two-stage model is similar to a Cholesky decomposition model (Neale and Cardon, 1992) and has been previously used to examine the relationship between initiation of substance use and progression to heavier use (e.g. Heath et al., 2002b). An important difference from the Cholesky is that, as part of data preparation, a missing data structure consistent with the DSM-IV is imposed on the data such that, as described above, individuals with values of 3+ on CD will not have ODD (i.e., ODD is set to missing). The missing data structure is considered Missing At Random (MAR; (Little and Rubin, 1987) because the probability of structural missing data on ODD is solely determined by values on CD. This model examines additive genetic as well as shared and non-shared environmental influences on both outcomes as well as the relationship between them. 95% likelihood-based confidence intervals were also computed.
We also extended the two-stage model to control for age by jointly modeling the probit regression of outcome (i.e., CD or ODD) on age and the genetic and environmental contributions to the residual variance and covariance among CD and ODD. In order to control for the age range in these data and following Knopik and colleagues (2009, 2005) we modeled age as a contrast coded covariate allowing for three groups: 11–14 years old, 15–18 years old, and 19+ years old. Models were fitted by maximum-likelihood using Mx (Neale et al., 2003), which is designed to handle MAR data and has been shown in simulations to appropriately recover the true polychoric correlation in two-stage models that incorporate structurally missing data (Heath et al., 2002b). Under this adjusted threshold model, genetic (additive) and environmental (shared and nonshared) parameter estimates were obtained after controlling for age.
Results
Twin pairs ranged in age from 11 to 23 years (mean= 15.15 years). The ODD profiles across categories of CD, as well as prevalence rates, are in Table 2. These rates are comparable to similar studies of community-based populations (e.g. Maughan et al., 2004, Nock et al., 2007).
Genetic analyses
Results of the two-stage model are in Table 4 and Figure 1b. The variance components and correlations obtained from fitting the full two-stage model confirm significant genetic influences on CD (a2=0.28, 95% CI=0.06–0.52) and ODD (a2=0.60, 95% CI=0.46–0.76), and are consistent with prior reports (Dick et al., 2005, Ehringer et al., 2006). Shared and nonshared environmental influences were also significant and important for both outcomes, a finding that moves beyond AE models reported for these phenotypes (Ehringer et al., 2006); see Table 4); although the magnitude of nonshared environmental effects differed between the two constructs. The estimated genetic correlation between CD and ODD was 0.52 (95% CI=0.18–1.0) implying that genetic influences on DSM-IV CD account for approximately 27% of the genetic variance in DSM-IV ODD. The estimated shared (rC=0.28) and nonshared (rE=0.03) environmental correlations between CD and ODD from the full two-stage model did not differ significantly from zero.
Table 4.
Two-stage model of CD and ODD using DSM-IV hierarchy: Model fit statistics, standardized variance components, and genetic and environmental correlations. All models are adjusted for age.
| Model | Parameters | Comparison to full model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD | ODD | Correlations | ||||||||||
| a2 | c2 | e2 | a2 | c2 | e2 | rA | rC | rE | Δχ2 | df | p | |
| 1. Full two-stage ACE | .28 (.06–.52) | .31 (.09–.50) | .41 (.35–.48) | .60 (.46–.76) | .29 (.13–.43) | .11 (.09–.14) | .52 (.18–1.00) | .28 (−.23–.76) | .03 (−.12–.18) | -- | -- | -- |
| 2. Two-stage AE Model | .61 (.55–.67) | -- | .39 (.33–.45) | .89 (.86–.91) | -- | .11 (.09–.14) | .40 (.31.48) | -- | .03 (−.11–.18) | 19.01 | 3 | <.001 |
| 3. Two-stage CE Model | -- | .54 (.47–.59) | .46 (.41–.52) | -- | .78 (.74–.81) | .22 (.19–.26) | -- | .41 (.32–.49) | .15 (.05–.26) | 106.5 | 3 | <.001 |
| 4. Two-stage Orth A (No A cov): Test of genetic distinctness | .24 (.02–.45) | .34 (.16–.53) | .42 (.35–.49) | .58 (.44–.73) | .31 (.16–.44) | .11 (.09–.14) | -- | .81 (.64–1.00) | .12 (−.02–.25) | 8.65 | 1 | .003 |
| 5. Two-stage Orth C (No C cov): Test of shared E distinctness | .32 (.11–.55) | .27 (.07–.45) | .41 (.35–.48) | .63 (.49–.78) | .26 (.11–.40) | .11 (.09–.15) | .67 (.46–1.00) | -- | .01 (−.14–.15) | 1.45 | 1 | .229 |
| 6. Two-stage Unidim A: Test of complete genetic overlap | .11 (.04–.26) | .45 (.30–.55) | .44 (.39–.51) | .59 (.46–.65) | .29 (.22–.43) | .12 (.09–.14) | 1.0 | .15 (−.30–.50) | .01 (−.15–.13) | 3.03 | 1 | .082 |
| 7. Two-stage Unidim C: Test of complete shared E overlap | .50 (.18–.66) | .11 (.06–.39) | .39 (.33–.36) | .62 (.33–.86) | .27 (.03–.42) | .11 (.09–.14) | .23 (−.03–.58) | 1.0 | .06 (−.10–.21) | 6.65 | 1 | .01 |
| 8. Two-stage Unidim A + Orth C:Test of complete genetic overlap but specific shared E effects | .15 (.09–.23) | .41 (.32–.48) | .44 (.38–.50) | .61 (.47–.77) | .27 (.12–.41) | .11 (.09–.14) | 1.0 | -- | −.02 (−.16–.30) | 3.644 | 2 | .162 |
We proceeded to formally test whether DSM-IV CD and ODD can be considered etiologically distinct by fitting a series of sub-models to the data (Table 4). Model 1 is our full two-stage model described above and the model to which all submodels were compared. Models 2 and 3 dropped shared environmental effects and additive genetic influences, respectively, and did not provide a better fit (AE model (Model 2): Δχ2=9.01, df=3, p<0.001; CE model (Model 3): Δχ2=106.5, df=3, p<0.001). Model 4 tested an orthogonal genetic liability model in which the genetic covariance between CD and ODD is set to zero (i.e., rA=0 or path a21=0). This model fit the data poorly (Δχ2=8.65, df=1, p=0.003); however, Model 5, which tested an orthogonal shared environmental liability model (rC=0 or c21=0) provided a more parsimonious fit to the data (Δχ2=1.45, df=1, p=0.229). We then fit two unidimensional models (Models 6 and 7) in which the specific genetic (model 6, a22=0) and shared environmental (model 7, c22=0) paths were dropped. This unidimensional model tests whether all genetic (Model 6) or environmental influences (Model 7) on ODD are shared with CD. Model 6 did not result in a significant detriment of fit (Δχ2=3.03, df=1, p=0.082) and Model 7 fit the data poorly (Δχ2=6.65, df=1, p=0.01). Finally, we fit a model that combined unidimensional genetic effects (Model 6) with orthogonal shared environmental effects (Model 5). This model (Model 8) also fit well (Δχ2=3.64, df=2, p=0.162) and suggests that all of the genetic influences on ODD might be shared with CD, while shared environmental effects are specific to each disorder.
In order to compare patterns of results from the two-stage model with the more common practice of analyzing symptom counts, we also ran a bivariate model using DSM-IV CD and ODD symptom counts (Table 5). This model included all individuals and ignored DSM-IV hierarchy. Overall, model fitting results were similar. The correlational structure (rA, rC, and rE) was also highly similar whether modeled using DSM-IV criteria or using symptom counts; however, variance component estimates and confidence intervals for ODD (which is defined differently between these two sets of models) did differ, with genetic effects accounting for more of the variance when defined using DSM-IV criteria. Thus, our inferences about genetic effects on ODD change dependent on how ODD is defined. This information could be important for future gene identification efforts in terms of how to model these behaviors to be most informative for analyses.
Table 5.
Model of CD and ODD using DSM-IV symptom counts: Model fit statistics, standardized variance components, and genetic and environmental correlations. All models are adjusted for age.
| Model | Parameters | Comparison to full model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD | ODD | Correlations | ||||||||||
| a2 | c2 | e2 | a2 | c2 | e2 | rA | rC | rE | Δχ2 | df | p | |
| 1. Full ACE | .18 (.17–.50) | .40 (.28–.52) | .42 (.38–.46) | .33 (.16–.50) | .16 (.01–.29) | .51 (.47–.56) | .53 (.10–1.00) | .17 (−.29–.63) | .09 (.02–.15) | -- | -- | -- |
| 2. AE Model | .61 (.57–.64) | -- | .39 (.36–.39) | .50 (.45–.54) | -- | .50 (.46–.55) | .30 (.27.38) | -- | .09 (.03–.15) | 41.42 | 3 | <.001 |
| 3. CE Model | -- | .55 (.51–.58) | .45 (.42–.49) | -- | .42 (.38–.46) | .58 (.54–.62) | -- | .29 (.22–.37) | .13 (.08–.18) | 24.24 | 3 | <.001 |
| 4. Orth A (No A cov): Test of genetic distinctness | .15 (.02–.28) | .43 (.31–.54) | .42 (.38–.47) | .29 (.13–.40) | .19 (.04–.32) | .52 (.47–.57) | -- | .51 (.48–.52) | .13 (.07–.18) | 5.58 | 1 | .018 |
| 5. Orth C (No C cov): Test of shared E distinctness | .19 (.07–.32) | .39 (.28–.50) | .42 (.38–.46) | .34 (.18–.51) | .14 (.00–.26) | .51 (.47–.56) | .66 (.43–1.00) | -- | .08 (.01–.09) | 0.77 | 1 | .382 |
| 6. Unidim A: Test of complete genetic overlap | .10 (.10–.24) | .47 (.35–.55) | .43 (.39–.47) | .30 (.12–.48) | .18 (.03–.33) | .52 (.47–.57) | 1.0 | .02 (−.42–.36) | .06 (.002–.13) | 3.01 | 1 | .083 |
| 7. Unidim C: Test of complete shared E overlap | .18 (.05–.32) | .40 (.28–.51) | .42 (.38–.46) | .49 (.43–.54) | .01 (.00–.05) | .50 (.46–.55) | .48 (.05–.99) | 1.0 | .09 (.02–.15) | 4.27 | 1 | .04 |
| 8. Unidim A + Orth C:Test of complete genetic overlap but specific shared E effects | .30 (.14–.47) | .18 (.03–.31) | .52 (.45–.52) | .11 (.05–.22) | .46 (.45–.52) | .43 (.39–.47) | 1.0 | -- | .06 (.002–.12) | 3.02 | 2 | .221 |
Because prior work has examined the covariation among CD and ODD in the presence of ADHD and determined significant shared genetic, shared environmental, and non-shared environmental correlations between CD and ODD in the presence of ADHD (Tuvblad et al 2009), we ran a series of models that included ADHD. While results will be included in a follow-up report, our full model resulted in parameter estimates and correlations between CD and ODD (once the genetic and environmental structure of ADHD is modeled) that are entirely consistent with the current report. Specifically, once ADHD is controlled for, heritability estimates were 0.33 (0.12–0.57) and 0.64 (0.51–0.80) for CD and ODD, respectively. Shared environmental effects were estimated for CD at 0.26 (0.04–0.37) and for ODD at 0.24 (0.10–0.38). The genetic correlation was 0.57 (0.26–0.98) and shared environmental effects were not significantly correlated at 0.16 (−0.51–0.71).
Discussion
We examined whether DSM-IV CD and ODD are etiologically distinct or share the same underlying liability in a community-based sample of female twins. Results suggest that, in this sample, covariation among these disorders can largely be attributed to shared genetic influences (rA=0.52, 95% CI=0.18–1.00). The best fitting two-stage model allowed genetic influences to overlap completely and no shared environmental correlation between DSM-IV CD and ODD. Though replication is necessary, these findings suggest that DSM-IV ODD and CD should be conceptualized as manifesting primarily from the same underlying genetic vulnerability, with specific shared environmental effects contributing to individual differences in each disorder.
The combination CD/ODD phenotype
These findings suggest that summing symptom counts across these two disorders for the purposes of purely genetic analyses appears justified; however, formal testing of that assumption might be warranted as samples and research questions may differ. In contrast to some earlier studies (e.g., (Tuvblad et al., 2009), we also find significant specific (i.e., uncorrelated) shared environmental effects that contribute to each behavior (i.e., shared environmental influences could not be dropped from the model, but rC could be set to zero). This would suggest that, for analyses that seek to examine shared environmental as well as genetic correlations of CD and ODD with other phenotypes, such as substance use, it would be important not to combine CD and ODD. In addition, explicating the particular shared environmental influences that specifically contribute to individual differences in each of these phenotypes could highlight potentially important therapeutic targets.
The role of ADHD
Our primary question involved the covariation of CD and ODD in the absence of ADHD. This is supported by prior studies that suggest that ADHD is a qualitatively distinct construct (Baving et al., 2006, Clark et al., 2000, Gadow and Nolan, 2002, Yoon et al., 2008) that co-segregates with disruptive behaviors as a unique trait (Jain et al., 2007). However, as noted above, prior work has modeled ADHD alongside CD and ODD (e.g., (Tuvblad et al., 2009). In an attempt to delineate these relationships, we extended our two-stage model to include ADHD and our full model yielded results consistent with results reported here, suggesting that our results are robust to the inclusion of comorbid ADHD. It will be critical to extend these findings by including other correlated behaviors in the externalizing or behavioral disinhibition spectrum (i.e., novelty seeking or substance use; (Young et al., 2009, Young et al., 2000).
Contributions and limitations
The use of a two-stage model to explore genetic and environmental overlap between ODD and CD provides a novel extension of an approach often utilized within the substance dependence literature (Heath et al., 2002b). This approach provides an innovative and alternative analysis of etiological influences while closely approximating the process undertaken by clinicians in diagnosing and, ultimately, treating disruptive behaviors. This close approximation to clinical diagnosis should not be undervalued. For example, in gene-identification efforts, where large samples are key, researchers might only have access to medical records with diagnostic categories. Thus, a better understanding of the etiological overlap of these disorders, as defined by DSM-IV, will be important to determining how best to model these phenotypes such that they are the most informative.
Another important contribution is the use of a large dataset ranging in age from 11 to 23 years. Previous studies have generally utilized younger samples (Dick et al., 2005, Lahey et al., 2009, Tuvblad et al., 2009), which might not be optimal because symptoms of CD do not typically develop until later in adolescence. Therefore, prior investigations using younger samples may not completely represent the diagnostic populations they intended to capture.
Our findings have clinical implications and suggest that treating ODD and CD symptoms distinctly (and per DSM-IV criteria), may not be warranted. This is supported by reports showing that the DSM-IV ODD criteria may miss clinically impaired children (Burke et al., 2010, Rowe et al., 2005). Taken together, these findings support a more continuous diagnostic approach of ODD and CD over the DSM-IV’s purely hierarchical rule (see Burke, et al., 2010 for a more complete discussion).
Limitations
First, our sample was entirely female. Thus, we could not examine gender differences. While prior literature suggests that the etiological structure between CD and ODD does not differ by sex (e.g. (Dick et al., 2005, Eaves et al., 2000), these prior investigations did not model DSM-IV defined diagnoses. Further, evidence suggests that both disorders are more common among males (APA, 1994). Therefore, it is important that these questions are tested in male and/or mixed gender samples. Second, future research considering longitudinal extensions of this two-stage model is also warranted. For example, ODD is often thought to be a precursor to CD. Given that ODD and CD were assessed concurrently in our sample, we could not directly model this developmental question.
This study is the first to incorporate a two-stage model to explore shared genetic and/or environmental overlap among these disorders as defined by DSM-IV. This is also one of the few investigations to employ an older age-cohort and to explore potential overlap between CD and ODD in lieu of including ADHD in the statistical model. Replication and extension of these findings will be important for advancing the understanding of the pathogenesis and, ultimately, treatment of these disorders.
Acknowledgments
This work supported by NIH grants: DA17671 (Knopik), AA07728 (Heath), AA09022 (Heath), AA11998 (Heath), HD049024 (Heath), AA017688 (Heath). Dr. Bidwell is supported by K23DA033302. Dr. Flessner is supported by R01 DA023134 (Knopik). Dr. Nugent is supported by K01 MH087240.
References
- APA. Diagnostic and statistical manual of mental disorders. 4. Washington D.C: 1994. [Google Scholar]
- Baving L, Rellum T, Laucht M, Schmidt MH. Children with oppositional-defiant disorder display deviant attentional processing independent of ADHD symptoms. Journal of Neural Transmission. 2006;113:685–93. doi: 10.1007/s00702-005-0345-x. [DOI] [PubMed] [Google Scholar]
- Biederman J, Ball SW, Mick E, Monuteaux MC, Kaiser R, Bristol E, Faraone SV. Informativeness of maternal reports on the diagnosis of ADHD: an analysis of mother and youth reports. Journal of Attention Disorders. 2007;10:410–7. doi: 10.1177/1087054706295656. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–58. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Burke JD, Waldman I, Lahey BB. Predictive validity of childhood oppositional defiant disorder and conduct disorder: implications for the DSM-V. Journal of Abnormal Psychology. 2010;119:739–51. doi: 10.1037/a0019708. [DOI] [PubMed] [Google Scholar]
- Burt SA, Krueger RF, McGue M, Iacono W. Parent-child conflict and the comorbidity among childhood externalizing disorders. Archives of General Psychiatry. 2003;60:505–13. doi: 10.1001/archpsyc.60.5.505. [DOI] [PubMed] [Google Scholar]
- Burt SA, Krueger RF, McGue M, Iacono WG. Sources of covariation among attention-deficit/hyperactivity disorder, oppositional defiant disorder, and conduct disorder: the importance of shared environment. Journal of Abnormal Psychology. 2001;110:516–25. doi: 10.1037/0021-843X.110.4.516. [DOI] [PubMed] [Google Scholar]
- Clark C, Prior M, Kinsella GJ. Do executive function deficits differentiate between adolescents with ADHD and oppositional defiant/conduct disorder? A neuropsychological study using the Six Elements Test and Hayling Sentence Completion Test. Journal of Abnormal Child Psychology. 2000;28:403–14. doi: 10.1023/a:1005176320912. [DOI] [PubMed] [Google Scholar]
- Dick DM, Viken RJ, Kaprio J, Pulkkinen L, Rose RJ. Understanding the covariation among childhood externalizing symptoms: genetic and environmental influences on conduct disorder, attention deficit hyperactivity disorder, and oppositional defiant disorder symptoms. Journal of Abnormal Child Psychology. 2005;33:219–29. doi: 10.1007/s10802-005-1829-8. [DOI] [PubMed] [Google Scholar]
- Eaves L, Rutter M, Silberg JL, Shillady L, Maes H, Pickles A. Genetic and environmental causes of covariation in interview assessments of disruptive behavior in child and adolescent twins. Behavior Genetics. 2000;30:321–34. doi: 10.1023/a:1026553518272. [DOI] [PubMed] [Google Scholar]
- Ehringer MA, Rhee SH, Young S, Corley R, Hewitt JK. Genetic and environmental contributions to common psychopathologies of childhood and adolescence: a study of twins and their siblings. Journal of Abnormal Child Psychology. 2006;34:1–17. doi: 10.1007/s10802-005-9000-0. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Nolan EE. Differences between preschool children with ODD, ADHD, and ODD+ADHD symptoms. Journal of Child Psychology and Psychiatry. 2002;43:191–201. doi: 10.1111/1469-7610.00012. [DOI] [PubMed] [Google Scholar]
- Heath AC, Howells W, Bucholz KK, Glowinski AL, Nelson EC, Madden PA. Ascertainment of a mid-western US female adolescent twin cohort for alcohol studies: assessment of sample representativeness using birth record data. Twin Research and Human Genetics. 2002a;5:107–12. doi: 10.1375/1369052022974. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PA. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Research and Human Genetics. 2002b;5:113–24. doi: 10.1375/1369052022983. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94:1361–70. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Jain M, Palacio LG, Castellanos FX, Palacio JD, Pineda D, Restrepo MI, Munoz JF, Lopera F, Wallis D, Berg K, Bailey-Wilson JE, Arcos-Burgos M, Muenke M. Attention-deficit/hyperactivity disorder and comorbid disruptive behavior disorders: evidence of pleiotropy and new susceptibility loci. Biological Psychiatry. 2007;61:1329–39. doi: 10.1016/j.biopsych.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Patrick CJ. Familial influences on conduct disorder reflect 2 genetic factors and 1 shared environmental factor. JAMA Psychiatry. 2013;70:78–86. doi: 10.1001/jamapsychiatry.2013.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Archives of General Psychiatry. 2003;60:709–17. doi: 10.1001/archpsyc.60.7.709. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Bucholz KK, Madden PA, Waldron M. Genetic and environmental influences on externalizing behavior and alcohol problems in adolescence: a female twin study. Pharmacology Biochemistry and Behavior. 2009;93:313–21. doi: 10.1016/j.pbb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Sparrow EP, Madden PA, Bucholz KK, Hudziak JJ, Reich W, Slutske WS, Grant JD, McLaughlin TL, Todorov A, Todd RD, Heath AC. Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: a female twin study. Psychological Medicine. 2005;35:625–35. doi: 10.1017/s0033291704004155. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Van Hulle CA, Rathouz PJ, Rodgers JL, D’Onofrio BM, Waldman ID. Are oppositional-defiant and hyperactive-inattentive symptoms developmental precursors to conduct problems in late childhood?: genetic and environmental links. Journal of Abnormal Child Psychology. 2009;37:45–58. doi: 10.1007/s10802-008-9257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little R, Rubin D. Statistical Analysis with Missing Data. John Wiley & Sons; New York: 1987. [Google Scholar]
- Loeber R, Burke JD, Lahey BB, Winters A, Zera M. Oppositional defiant and conduct disorder: a review of the past 10 years, part I. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1468–84. doi: 10.1097/00004583-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Maughan B, Rowe R, Messer J, Goodman R, Meltzer H. Conduct disorder and oppositional defiant disorder in a national sample: developmental epidemiology. Journal of Child Psychology and Psychiatry. 2004;45:609–21. doi: 10.1111/j.1469-7610.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- Nadder TS, Rutter M, Silberg JL, Maes HH, Eaves LJ. Genetic effects on the variation and covariation of attention deficit-hyperactivity disorder (ADHD) and oppositional-defiant disorder/conduct disorder (Odd/CD) symptomatologies across informant and occasion of measurement. Psychological Medicine. 2002;32:39–53. doi: 10.1017/s0033291701004792. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes H. Mx: Statistical Modeling. VCU Box 900126, Richmond, VA: Department of Psychiatry; 2003. (Version 6th Edition) [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Kluwer; Dordrecht, The Netherlands: 1992. [Google Scholar]
- Nichols RC, Bilbro WC., Jr The diagnosis of twin zygosity. Acta Genetica Statistica Medica. 1966;16:265–75. doi: 10.1159/000151973. [DOI] [PubMed] [Google Scholar]
- Nock MK, Kazdin AE, Hiripi E, Kessler RC. Lifetime prevalence, correlates, and persistence of oppositional defiant disorder: results from the National Comorbidity Survey Replication. Journal of Child Psychology and Psychiatry. 2007;48:703–13. doi: 10.1111/j.1469-7610.2007.01733.x. [DOI] [PubMed] [Google Scholar]
- Olsson U. Maximum likelihood estimation of the polychoric correlation coefficient. Psychometrika. 1979;44:443–460. [Google Scholar]
- Reich W. Diagnostic interview for children and adolescents (DICA) Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Rettew DC, Rebollo-Mesa I, Hudziak JJ, Willemsen G, Boomsma DI. Non-additive and additive genetic effects on extraversion in 3314 Dutch adolescent twins and their parents. Behavior Genetics. 2008;38:223–33. doi: 10.1007/s10519-008-9192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Willcutt EG, Hartman CA, Pennington BF, DeFries JC. Test of alternative hypotheses explaining the comorbidity between attention-deficit/hyperactivity disorder and conduct disorder. Journal of Abnormal Child Psychology. 2008;36:29–40. doi: 10.1007/s10802-007-9157-9. [DOI] [PubMed] [Google Scholar]
- Rothen S, Vandeleur CL, Lustenberger Y, Jeanpretre N, Ayer E, Gamma F, Halfon O, Fornerod D, Ferrero F, Preisig M. Parent-child agreement and prevalence estimates of diagnoses in childhood: direct interview versus family history method. International Journal of Methods in Psychiatric Research. 2009;18:96–109. doi: 10.1002/mpr.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe R, Maughan B, Costello EJ, Angold A. Defining oppositional defiant disorder. Journal of Child Psychology and Psychiatry. 2005;46:1309–16. doi: 10.1111/j.1469-7610.2005.01420.x. [DOI] [PubMed] [Google Scholar]
- Silberg J, Rutter M, Meyer J, Maes H, Hewitt J, Simonoff E, Pickles A, Loeber R, Eaves L. Genetic and environmental influences on the covariation between hyperactivity and conduct disturbance in juvenile twins. Journal of Child Psychology and Psychiatry. 1996;37:803–16. doi: 10.1111/j.1469-7610.1996.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Tuvblad C, Zheng M, Raine A, Baker LA. A common genetic factor explains the covariation among ADHD ODD and CD symptoms in 9–10 year old boys and girls. Journal of Abnormal Child Psychology. 2009;37:153–67. doi: 10.1007/s10802-008-9278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron M, Madden PA, Nelson EC, Knopik VS, Glowinski AL, Grant JD, Lynskey MT, Jacob T, Sher KJ, Bucholz KK, Heath AC. The interpretability of family history reports of alcoholism in general community samples: findings in a midwestern U.S. twin birth cohort. Alcohol: Clinical and Experimental Research. 2012;36:1091–8. doi: 10.1111/j.1530-0277.2011.01698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HR, Xie M, Thompson W, Loeber R, Stouthamer-Loeber M. Psychopathology as a predictor of adolescent drug use trajectories. Psychology of Addictive Behavior. 2001;15:210–8. [PubMed] [Google Scholar]
- WHO, W. H. O. International Statistical Classification of Diseases and Related Health Problems. Geneva, Switzerland: 1992. (10th Revision ed.) [Google Scholar]
- Yoon HH, Iacono WG, Malone SM, Bernat EM, McGue M. The effects of childhood disruptive disorder comorbidity on P3 event-related brain potentials in preadolescents with ADHD. Biological Psychology. 2008;79:329–36. doi: 10.1016/j.biopsycho.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118:117–30. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics. 2000;96:684–95. [PubMed] [Google Scholar]