Fig. 5. IRBIT-mediated synergistic activation of epithelial fluid and HCO3− secretion by the cAMP and Ca2+ signaling pathways.
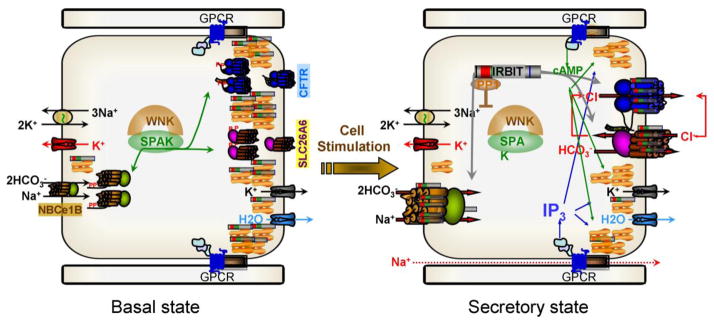
In the resting state the WNK/SPAK kinases associate with the transporters and SPAK phosphorylates NBCe1-B AID, Slc26a6 STAS domain and CFTR R domain to sequester most of them in intracellular organelles. Al low cytoplasmic IP3 IRBIT is bound to the IP3 receptors that in secretory glands are clustered at the apical pole. When the cells are stimulated with a combination of physiological concentrations of IP3 and cAMP generating agonists, PKA phosphorylates the IP3Rs to facilitate release of IRBIT from the IP3Rs by IP3 binding. IRBIT recruits PP1 to the transporters to dephosphorylate them and at the SPAK phosphorylation sites and target them to the plasma membrane. IRBIT remains bound to the transporters AIDs to relieve their constitutive inhibition resulting in activation of the transporters and of fluid and electrolyte secretion.
