Figure 1.
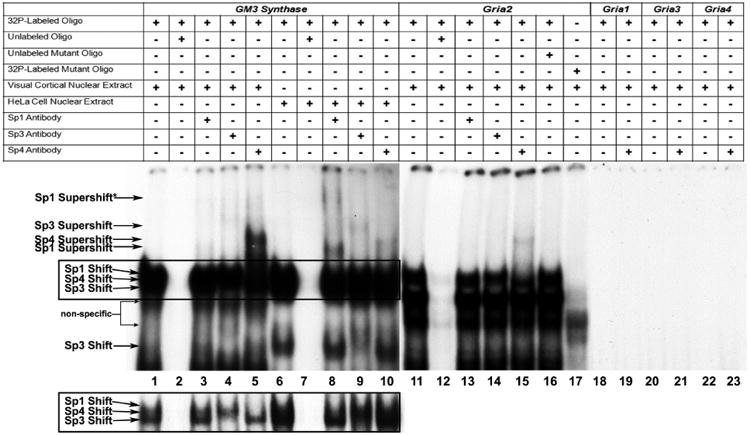
In vitro binding of Sp factors to the GM3 Synthase and Gria2 promoters using EMSA and supershift assays. All lanes contain 32P-labeled oligonucleotides and are labeled by a “+” or a “−“sign depending on whether they also contain mouse visual cortical or HeLa nuclear extract, excess oligos or mutant oligos (unlabeled), and Sp1, Sp3, or Sp4 antibodies. Sp1, Sp3, or Sp4 shift, supershift, and non-specific complexes are indicated by arrows. The positive control for Sp factor binding was GM3 Synthase. Specific Sp1, 3, or 4 shift bands were revealed upon incubation with cortical (lanes 1-5) or HeLa (lanes 5-10) nuclear extract (lanes 1 and 6, respectively). Excess unlabeled competitor competed the shift bands (lanes 2 and 7). The addition of Sp1 antibody did not yield a supershift band with cortical nuclear extract (lane 3) but gave two specific supershift bands with HeLa nuclear extract (lane 8), corresponding to the presence of tandem Sp binding. The addition of Sp3 antibody yielded a faint specific supershift band with cortical nuclear extract (lane 4) and a stronger supershift band with HeLa nuclear extract (lane 9). The addition of Sp4 antibody yielded a very strong supershift band with cortical nuclear extract (lane 5) and a much fainter supershift band with HeLa nuclear extract (lane 10). The relative levels of the Sp1, Sp3, and Sp4 supershift bands in mouse cortical and HeLa nuclear extract corresponds to the relative levels of these transcription factors in these tissue and cell types.
As Sp1, 3, and 4 all recognize the same cis motif, their combined binding yielded a rather thick shift band. However, with shorter gel exposure (boxed lanes 1 to 10 highlighted in the lower left), the individual shift bands can sometimes be dissociated. As shown in lane 1 in the inset, cortical nuclear extract did not reveal any detectable Sp1 shift band, but did show distinct Sp3 and Sp4 bands. The Sp3 shift band disappeared when it was supershifted (lane 4). Likewise, the Sp4 shift band disappeared when it was supershifted (lane 5). With HeLa nuclear extract, the rather abundant presence of both Sp1 and Sp3 caused the bands to merge even with lighter exposure (lane 6). However, the upper portion (Sp1) disappeared when supershifted with Sp1 antibody (lane 8), and the lower portion (Sp3) became lighter when supershifted with Sp3 antibody (lane 9). No change was detectable with anti-Sp4 antibody (lane 10), indicating that HeLa nuclear extract did not contain detectable Sp4.
Incubation of cortical nuclear extract with the Gria2 probe yielded a specific Sp4 shift band (lane 11) which was competed out with the addition of excess cold probes (lane 12). The addition of Sp4 anitbody yielded a specific supershift band (lane 15), but the addition of Sp1 and Sp3 antibodies did not (lanes 13 and 14). The addition of excess unlabeled mutant Gria2 probes did not compete out the shift reaction (lane 16). Labeled Gria2 probes with mutant Sp sites did not yield specific Sp shift bands (lane 17). Labeled Gria1, Gria3, and Gria4 probes did not yield specific Sp shift (lanes 18, 20, and 22, respectively) or Sp4 supershift bands (lanes 19, 21, and 23, respectively).
