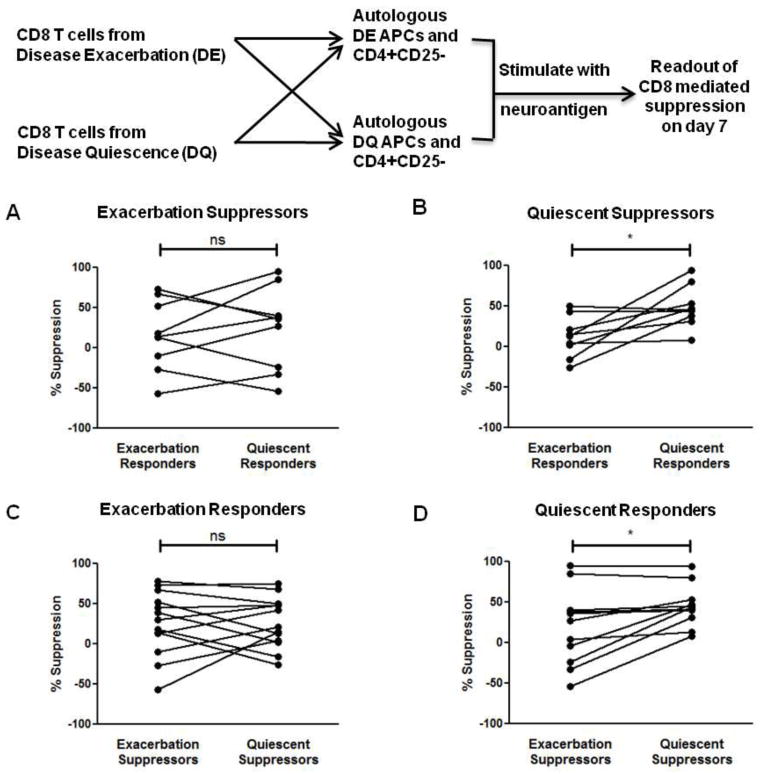
Figure 5. Loss of CD4+ T cell suppressibility is intrinsic to CD8+ Tregs and CD4+ T cells.
(A) Exacerbation-derived CD8+ T cells were isolated from MS patients and used in suppression assays with either exacerbation or quiescence-derived autologous CD4+CD25− T cell responders and APCs. No significant difference in exacerbation-derived CD8+ T cell suppressive ability was seen between the two responder sets (ns). (B) Quiescent CD8+ T cells display enhanced suppressive ability. Quiescence-derived CD8+ T cells were used in suppression assays with either exacerbation or quiescence-derived autologous CD4+CD25− T cell responders and APCs. Quiescence-derived CD8+ T cells showed significantly increased suppressive ability against quiescence-derived autologous CD4+CD25− T cell responders. (C) Exacerbation-derived CD4+CD25− T cells and APCs were used in suppression assays with either exacerbation or quiescence-derived autologous CD8+ T cells. No significant difference in exacerbation-derived CD4+CD25− T cell suppressibility was seen by between the two sets of CD8+ T cells (ns). (D) Quiescence-derived CD4+CD25− T cells and APCs were used in suppression assays with either exacerbation or quiescence-derived autologous CD8+ T cells. Quiescence-derived CD4+CD25− T cells were significantly more amenable to quiescence-derived CD8+ T cell-mediated suppression. *p < 0.05; PBMCs were obtained from 7 RRMS patients during both disease quiescence and relapse.