Figure 3. Preparation of PDZ Domain-HaloTag-HaloLink Affinity Resin.
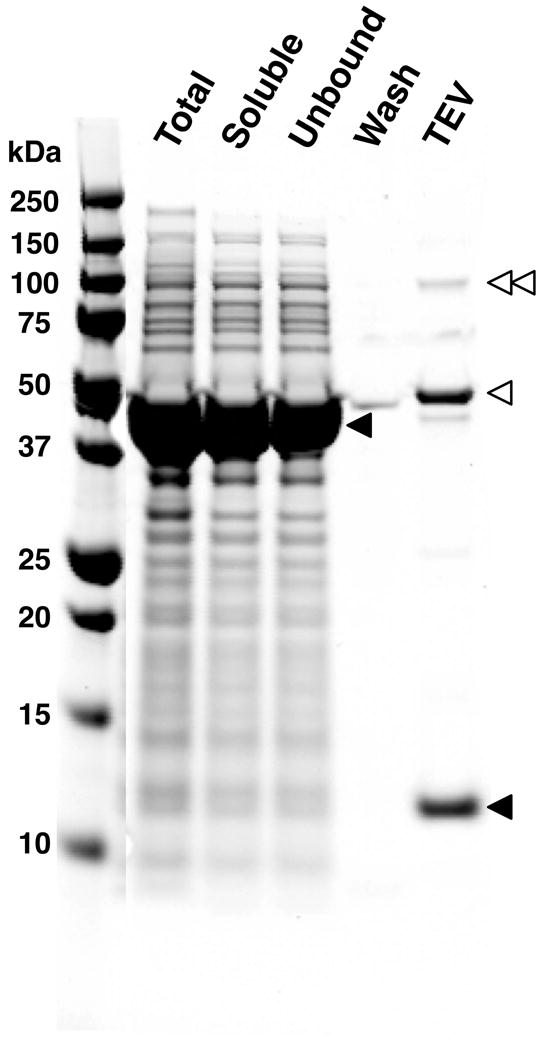
A fusion protein in which the PDZ3 domain was fused to the HaloTag was prepared as described in Methods. The PDZ3 Domain Affinity Resin was synthesized by coupling of the fusion protein to HaloLink Resin. The derivatized resin was washed extensively to remove contaminants. To measure the binding capacity of the resin, bound PDZ domains were released from an aliquot of the resin by digestion with TEV protease and quantified as described in Methods. In this experiment, resin capacity (pmol PDZ domain /μl resin) was 350 μM. Total protein, soluble protein, unbound protein, wash and TEV protease-treated fractions from the preparation were run on a 4-12% gradient SDS-PAGE gel with Precision Plus Protein All Blue Standards and stained with Gel Code Blue. Single and double unfilled arrowheads indicate the positions of monomeric and dimeric TEV protease, respectively. The upper and lower filled arrowheads indicate the positions of the PDZ3 domain-HaloTag fusion protein and the free PDZ3 domain, respectively. The lighter band below the TEV protease monomer is a contaminant present in the TEV protease.
