Figure 4. Purification of Heterologously Expressed Neuronal Proteins with Endogenous PDZ Domain Ligands.
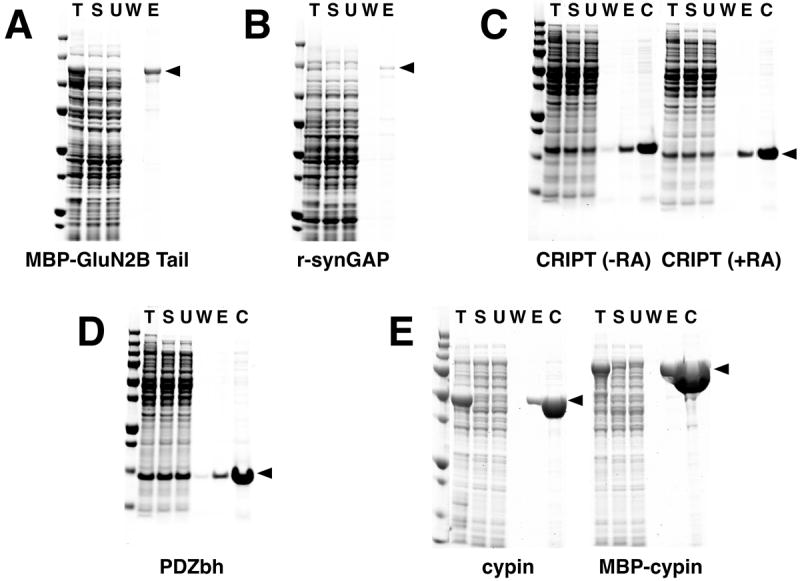
Six heterologously expressed neuronal proteins with naturally occurring PDZ domain ligands were purified on PDZ Domain Affinity Resins as described in Methods: (A) Purification of MBP-GluN2B Tail; (B) Purification of His-tagged r-synGAP; (C) Purification of CRIPT in the presence or absence of reducing agents (RA); (D) Purification of PDZbh; (E) Purification of cypin and MBP-cypin. MBP-GluN2B Tail and PDZbh were bound to PDZ2 Domain-HaloTag-HaloLink Resin and eluted with 400 μg/ml SIESDV peptide. Note that the ligand density of the PDZ2-resin used for purifications shown in (A) and (D) was 53 pmol/μl resin. Subsequent preparations of PDZ2-resin achieved the higher ligand density of 240-260 pmol/μl resin shown in Table 2. R-synGAP and CRIPT were bound to PDZ3 Domain-HaloTag-HaloLink Resin and eluted with 400 μg/ml YKQTSV peptide. Cypin and MBP-cypin were bound to tandem PDZ1+PDZ2 Domain-HaloTag-HaloLink Resin and eluted with 400 μg/ml SIESDV peptide. In the experiments shown in C and D the resin was incubated with an amount of recombinant POI in excess of the resin capacity. Fractions from each purification and Precision Plus Protein All Blue Standards were separated on a 4-12% gradient SDS-PAGE gel and stained with Gel Code Blue. T, Total protein; S, soluble protein; U, unbound protein; W, wash; E, peptide eluate; C, concentrated peptide eluate.
