Figure 5. Comparison of HaloTag-HaloLink and NHS linkage for Synthesis of PDZ Domain-Agarose Affinity Resin.
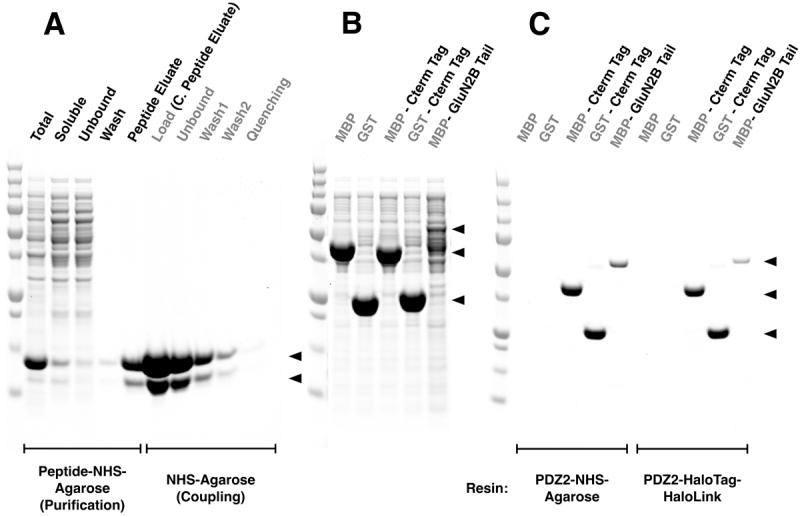
All samples and Precision Plus Protein All Blue Standards were fractionated by SDS-PAGE and protein was stained with Gel Code Blue.
(A) Purification of PDZ2 and coupling to NHS Agarose resin. The first 5 lanes show fractions from the purification of the PDZ2 domain on GluN2B ligand-NHS-Agarose as described in Methods: Total, bacterial extract; Soluble, extract after centrifugation; Unbound, column flow-through; Wash; Peptide Eluate, protein eluted with 400 μg/ml SIESDV peptide. The peptide was removed from the PDZ2 domain, as described in Methods, before linkage to NHS-Agarose. The next 5 lanes show fractions from coupling of the purified PDZ2 Domain to NHS-Agarose beads (as described in Methods): Load, concentrated peptide eluate that was incubated with NHS-Agarose; Unbound, supernatant after linkage; Wash1 & 2, two washes of the resin before quenching; Quenching, supernatant after quenching of unreacted NHS groups with ethanolamine. Upper and lower arrows indicate full length and truncated PDZ2 domain, respectively.
(B) Soluble lysate after heterologous expression of MBP, GST, their respective fusion proteins containing the 7-residue C-terminal PDZ2 Domain ligand Affinity Tag from GluN2B, and the entire GluN2B Tail fused to the C-terminus of MBP (see Methods).
(C) Purification of the fusion proteins shown in (B) on PDZ2-NHS-Agarose or on PDZ2 Domain-HaloTag-HaloLink resin. Soluble lysates were purified on the indicated Affinity Resins and eluted with 400 μg/ml SIESDV peptide, as described in Methods. The lysates containing MBP and GST fused to PDZ2 contained more protein than the capacity of the resin; thus these two columns were overloaded.
