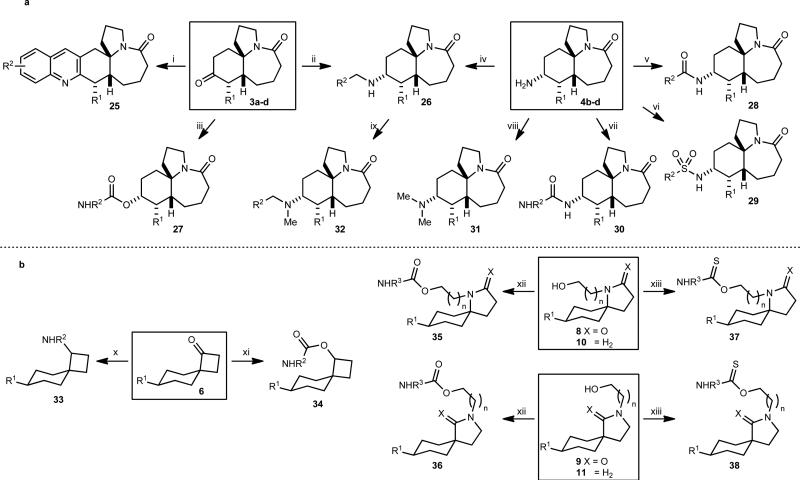
Figure 3. Library construction from Stemonaceae and cylindricine alkaloidinspired scaffolds.
(a) Ketone scaffolds 3a-d and amines 4b-d were converted into libraries of quinolines, amines, amides, sulfonamides, ureas and carbamates; the key scaffolds are indicated by boxes. (b) Ketone scaffolds 6, lactams 8 and 9, and amines 10 and 11 were converted into a series of libraries of amines, carbamates and thiocarbamates. A total of 499 unique structures, each obtained in >90% purity (HPLC, UV detector at 214 nm) and >20 mg quantities were obtained. Scaffold (number of final products obtained): 3a-d (131), 4c-d (191), 6 (112), 8 (19), 9 (12), 10 (32), 11 (2). (i) 2-Nitrobenzaldehyde, Fe0, 0.1 M aq HCl, EtOH, 85 °C; then 3a-d, KOH, 85 °C; (ii) amine, AcOH, Na(OAc)3BH, CH2Cl2, rt; (iii) a) L-Selectride, THF, −78 °C to rt, 90–95%, 9:1– ≥19:1 dr, (b) 4-nitrophenylchloroformate, pyridine, rt, 55–76%, (c) R2NH2, CH2Cl2, rt; (iv) R2CHO, AcOH, Na(OAc)3BH, CH2Cl2, rt; (v) R2CO2H, EDC, DMAP, CH2Cl2, rt; (vi) R2SO2Cl, Et3N, CH2Cl2, rt; (vii) R2N=C=O, PhMe, rt; (viii) H2C=O, HCO2H, 95 °C; (ix) H2C=O, AcOH, Na(OAc)3BH, CH2Cl2, rt; (x) R2NH2, DCE, microwave, 150 °C; (xi) a) NaBH4, THF, MeOH, rt, 94–97%, (b) R2N=C=O, Et3N, THF, rt; (xii) R2N=C=O, Et3N, THF, rt; (xiii) R2N=C=S, NaH, THF, rt.