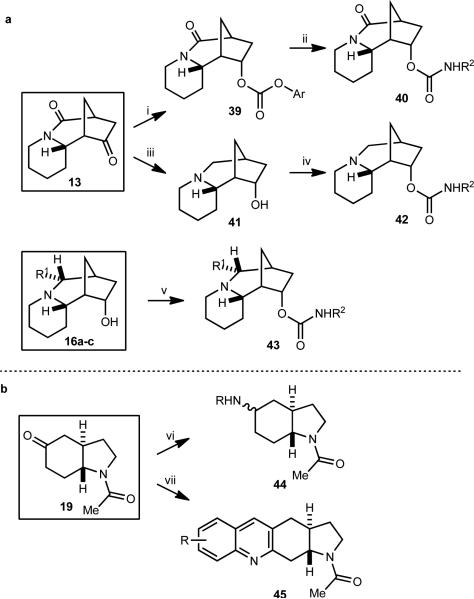
Figure 4. Library construction from sparteine and mesembrine-inspired scaffolds.
(a) Library construction from sparteine-inspired scaffolds led to carbamates generated both from lactam 13 directly or by first converting it to the amine-containing scaffold 41. Similar chemistry could be used on the additional alkyl-group-containing scaffolds 16a–c. (b) Amide scaffold 19 was converted into amine and quinoline libraries. A total of 132 unique structures, each obtained in >90% purity (HPLC, UV detector at 214 nm) and >10 mg quantities were obtained. Scaffold (number of final products obtained): 13 (44), 16a-c (52), 19 (36). (i) a) NaBH4, MeOH, rt, 88%, >19:1 dr, (b) 4-nitrophenyl chloroformate, pyridine, THF, rt, 95%; (ii) R2NH2, DCE, rt; (iii) LiAlH4, THF, reflux, 80%; (iv) R2N=C=O, MeCN, microwave, 110 °C; (v) R2N=C=O, THF, rt; (vi) RNH2, AcOH, Na(OAc)3BH, THF; (vii) 2-nitrobenzaldehyde, Fe0, 0.1M HCl, EtOH, 85 °C; then 19, KOH, 85 °C.