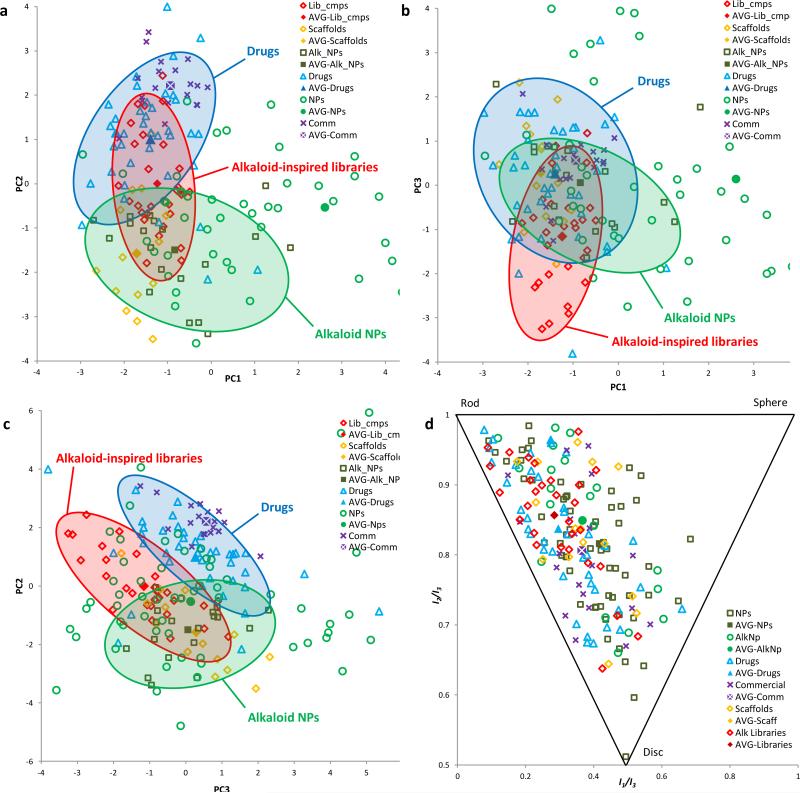
Figure 6. Cheminformatic analysis of alkaloid-inspired scaffolds and library members.
Structural and physiochemical properties of a representative selection of synthesized scaffolds (14 compounds, yellow diamonds) and library members (29 compounds, red diamonds) were compared with those of alkaloid NPs (20 compounds, green squares) and an established reference set48 of drugs (40 compounds, blue triangles), commercially available drug-like molecules (20 compounds, purple crosses) and NPs (60 compounds, green circles) using principal component analysis (PCA) and principal moment of inertia (PMI) analysis. The hypothetical average (mean) structure for each series is also plotted (AVG-). (a) PCA plot of PC1 v PC2. (b) PCA plot of PC1 v PC3. (c) PCA plot of PC2 v PC3. (d) PMI plot showing the 3-dimensional shape of the lowest energy conformer of each compound. The shaded red, green, and blue areas outline the regions of the plot where the majority of our alkaloid inspired libraries, alkaloid NPs, and drugs, respectively, are located.